4246 First Aid Kit by Honeywell Safety Products USA, INC 4246 FIRST AID KIT kit
4246 First Aid Kit by
Drug Labeling and Warnings
4246 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Eyewash Active ingredient
- Eyewash Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyewash Directions
- Eyewash Inactive ingredients
- Eyewash Questions
- Aspirin Active ingredient (in each tablet)
- Aspirin Purpose
- Aspirin Uses
-
Aspirin
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:are:
- age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout or arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- any new symptoms appear
If pregnant or breast-feeding,
If pregnant or breat-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- Aspirin Directions
- Aspirin Other information
- Aspirin Inactive ingredients
- Aspirin Questions or Comments
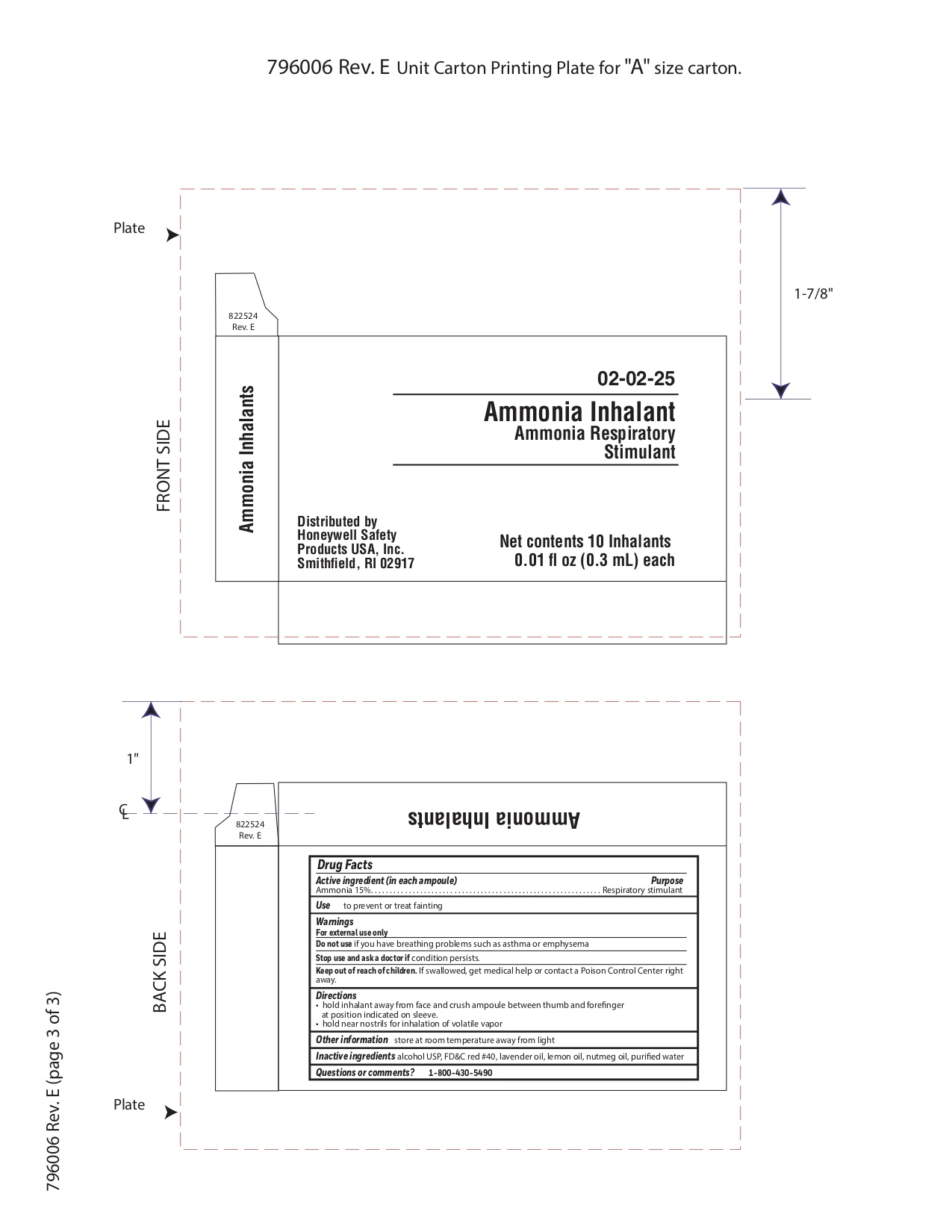
- Ammonia Active ingredient
- Ammonia Purpose
- Ammonia Uses
- Ammonia Warnings
- Ammonia Directions
- Ammonia Other information
- Ammonia Inactive ingredient
- Ammonia Questions or Comments?
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn Jel Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
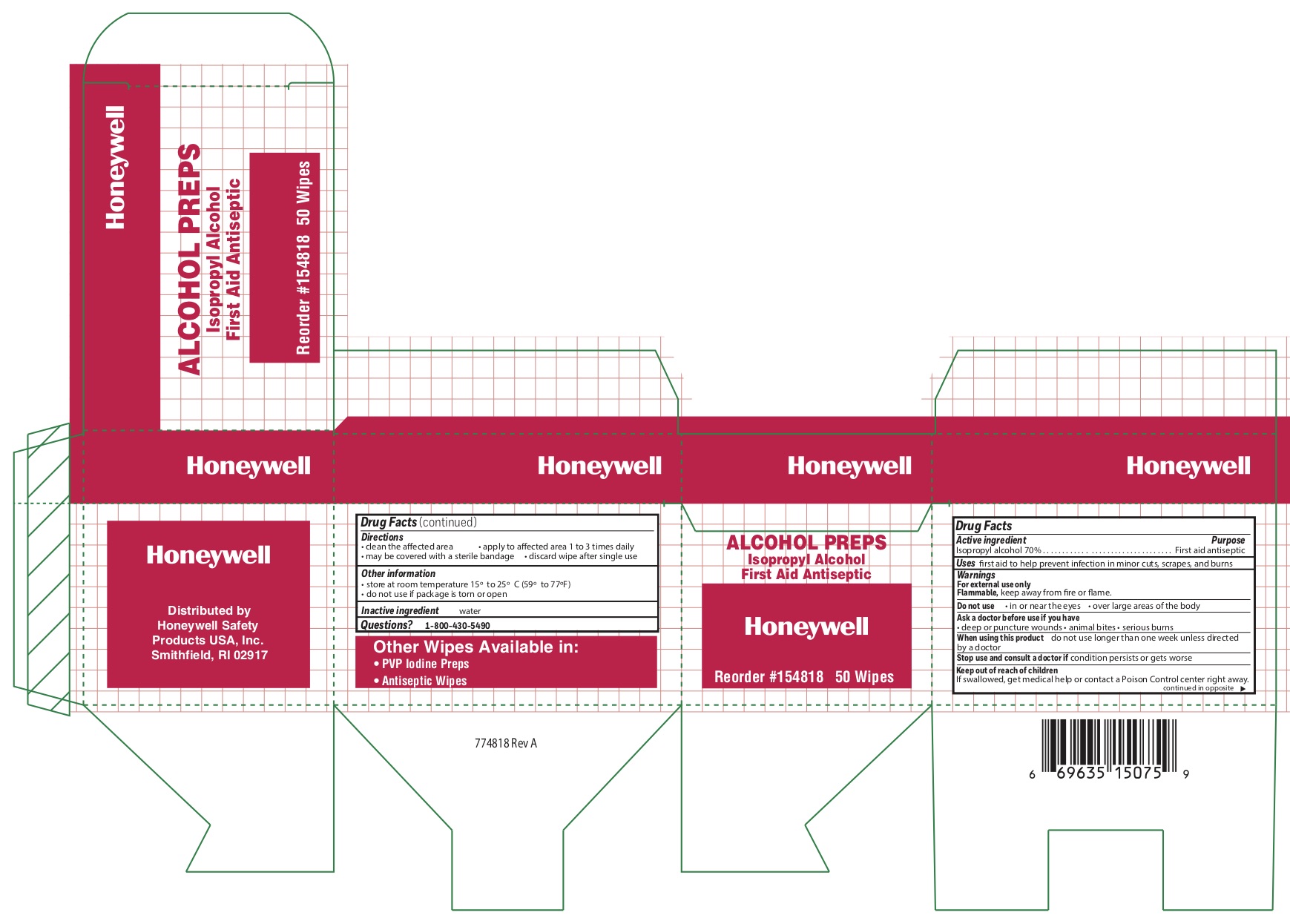
- Alcohol Wipes Active ingredient
- Alcohol Wipes Purpose
- Alcohol Wipes Uses
- Alcohol Wipes Warnings
- Alcohol Wipes Directions
- Alcohol Wipes Other information
- Alcohol Wipe Inactive ingredient
- Alcohol Wipe Questions
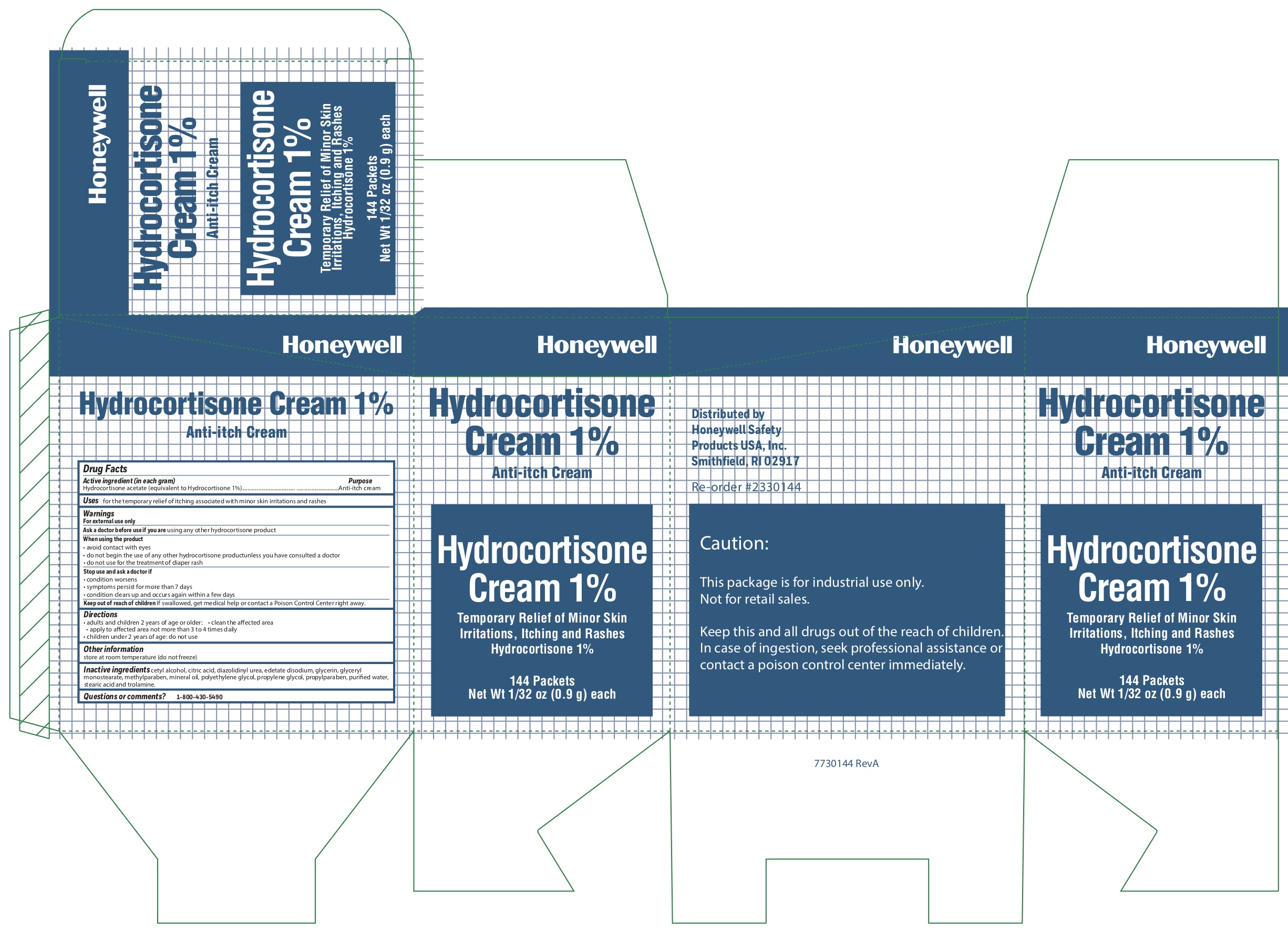
- Hydrocortisone Acitive ingredient (in each gram)
- Hydrocortisone Purpose
- Hydrocortisone Uses
-
Hydrocortisone
Warnings
For external use only
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
- Hydrocortisone Directions
- Hydrocortisone Other information
- Hydrocortisone Inactive ingredients
- Hydrocortisone Questions or Comments?
- PVP Wipe Active ingredient
- PVP Wipe Purpose
- PVP Wipe Uses
- PVP Wipe Warnings
- PVP Wipe Directions
- PVP Other information
- PVP Inactive ingredients
- PVP Questions and Comments?
- Burn Relief Active ingredient
- Burn Relief Spray Purpose
- Burn Spray Uses
- Burn Relief Spray Warnings
- Burn Relief Directions
- Burn Relief Spray Other information
- Burn Reelief Spray Inactive ingredients
- Burn Relief Spray Questions or Comments?
- Aypanal Active ingredient
- Aypanal Purpose
- Aypanal Uses
-
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin rash occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
-
Aypanal
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
- children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- children under 6 years
- consult a doctor
- Aypanal Other information
- Aypanal Inactive ingredients
- Aypanal Questions or Comments?
- Foille Ointment Active ingredient
- Foille Ointment Purpose
- Foille Ointment Uses
- Foille Ointment Warnings
- Foille Ointment Directions
- Foille Ointment Other information
- Foille Oinatment Inactive ingredients
-
4246
68100REC Kit Contents
1 3/4 X 3 PLAS 100/BOX
1 1X3 PLASTIC 100/BOX
1 KING SIZE 2 X 3 PLASTIC 50/BX
1 AMMONIA INHALANTS 10 PER
1 GAUZE COMPRESS, 1728 SQ IN 1
4 INSTANT COLD PACK 4" X 6"
3 BANDAGE COMP, 4" OFFSET, 1 PER
1 BURN JEL 1/8 OZ, 6 PER
1 ALCOHOL PREP PADS 10P
1 HYDROCORTISON,1.O%,1/32 OZ,10P
2 ADHESIVE TAPE W/P 1/2"X 5 YD
2 O/H PUMP BURN RELIEF 2 OZ ID G
2 GAUZE BANDAGE 1" x 2 YDS
1 FIRST AID GUIDE ASHI
1 EMERGENCY SURVIVAL BLANKET
6 TAPE ADHESIVE 1"X 5 YD PLSTC
2 GZE PADS STERILE 2"X 2" 10'S
2 GZE PADS STERILE 3"X 3" 10'S
2 GZE PADS STERILE 2"X 2" 25'S
5 CO-FLEX BAND 1"X5YD TAN 2/PACK
2 CO-FLEX BANDAGE 4"X5YDS TAN
1 PVP PREP PADS MEDIUM 100/BX
1 ASPIRIN IND PK 5 GR 2/ENV 100
1 212 4 OZ TUBE SKIN COND, 1 EA ID P
2 1 OZ, BUFF EYEWASH
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 180 EMPTY BLANK NO LOGO
1 TONGUE BLADES SR WRAPPED 6'S
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
2 SELF-ADH WRAP 3 X 5 YDS NORTH REV E
2 TRI BNDG NON WOVEN 40"X40"X56"
2 FOILLE BURN/F A OINT 1/2 OZ
8 EYE PADS STD OVAL STERILE
5 GAUZE PADS 4"X4" 12PLY
1 WOVEN KNUCKLE 8'S
1 FINGERTIP "T" 8/BX
1 TRNQT W/WEB STP&BKL 1
1 AYPANAL NON-ASP 25/2
- PRINCIPAL DISPLAY PANEL
- Aspirin Principal Display Panel
- Ammonia Principal Display Panel
- Burn Jel Principal Display Panel
- Alcohol Wipe Principal Display Panel
- Hydrocortisone Principal Display Panel
- PVP Principal Display Panel
- Burn Relief Spray Principal Display Panel
- Aypanal Principal Display Panel
- Foille Ointment Principal Display Panel
- 4246 Kit Label 68100REC
-
INGREDIENTS AND APPEARANCE
4246 FIRST AID KIT
4246 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4246 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4246-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 TUBE 28 g Part 2 10 PACKET 9 g Part 3 2 BOTTLE 60 mL Part 4 50 PACKET 100 Part 5 10 AMPULE 3 mL Part 6 2 BOTTLE, SPRAY 118 mL Part 7 10 POUCH 4 mL Part 8 100 POUCH 30 mL Part 9 6 PACKET 21 g Part 10 10 PACKET 9 g Part 11 25 PACKET 50 Part 1 of 11 BLISTEX FOILLE MEDICATED FIRST AID
benzocaine and chloroxylenol ointmentProduct Information Item Code (Source) NDC: 10157-9302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.1 g in 100 g BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) CERESIN (UNII: Q1LS2UJO3A) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) SODIUM BORATE (UNII: 91MBZ8H3QO) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CORN OIL (UNII: 8470G57WFM) EDETATE CALCIUM DISODIUM ANHYDROUS (UNII: 8U5D034955) EUGENOL (UNII: 3T8H1794QW) MALEIC ANHYDRIDE (UNII: V5877ZJZ25) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/05/2013 Part 2 of 11 HYDROCORTISONE
anti-itch creamProduct Information Item Code (Source) NDC: 0498-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) CETYL ALCOHOL (UNII: 936JST6JCN) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0801-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/15/2019 Part 3 of 11 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 4 of 11 ASPIRIN
aspirin tabletProduct Information Item Code (Source) NDC: 0498-0114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) MINERAL OIL (UNII: T5L8T28FGP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code FR21 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0114-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/18/2018 Part 5 of 11 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC: 0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 6 of 11 BURN RELIEF
lidocaine hydrochloride sprayProduct Information Item Code (Source) NDC: 0498-0221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 24.64 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) HYPROMELLOSES (UNII: 3NXW29V3WO) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0221-59 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/18/2018 Part 7 of 11 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 8 of 11 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 9 of 11 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) DIPROPYLENE GLYCOL (UNII: E107L85C40) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/18/2018 Part 10 of 11 HYDROCORTISONE
anti-itch cream ointmentProduct Information Item Code (Source) NDC: 0498-0800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) CETYL ALCOHOL (UNII: 936JST6JCN) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0800-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/06/2013 10/15/2019 Part 11 of 11 AYPANAL NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC: 0498-2001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code circle;U Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-2001-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 01/02/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 manufacture(10157-9302) Establishment Name Address ID/FEI Business Operations James Alexander 040756421 manufacture(0498-3334) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4246) Establishment Name Address ID/FEI Business Operations Ultra Seal Corporation 085752004 manufacture(0498-0114, 0498-2001) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0221, 0498-0203, 0498-0800, 0498-0801) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0143) Establishment Name Address ID/FEI Business Operations Sion Medical Biotext 532775194 manufacture(0498-0121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.