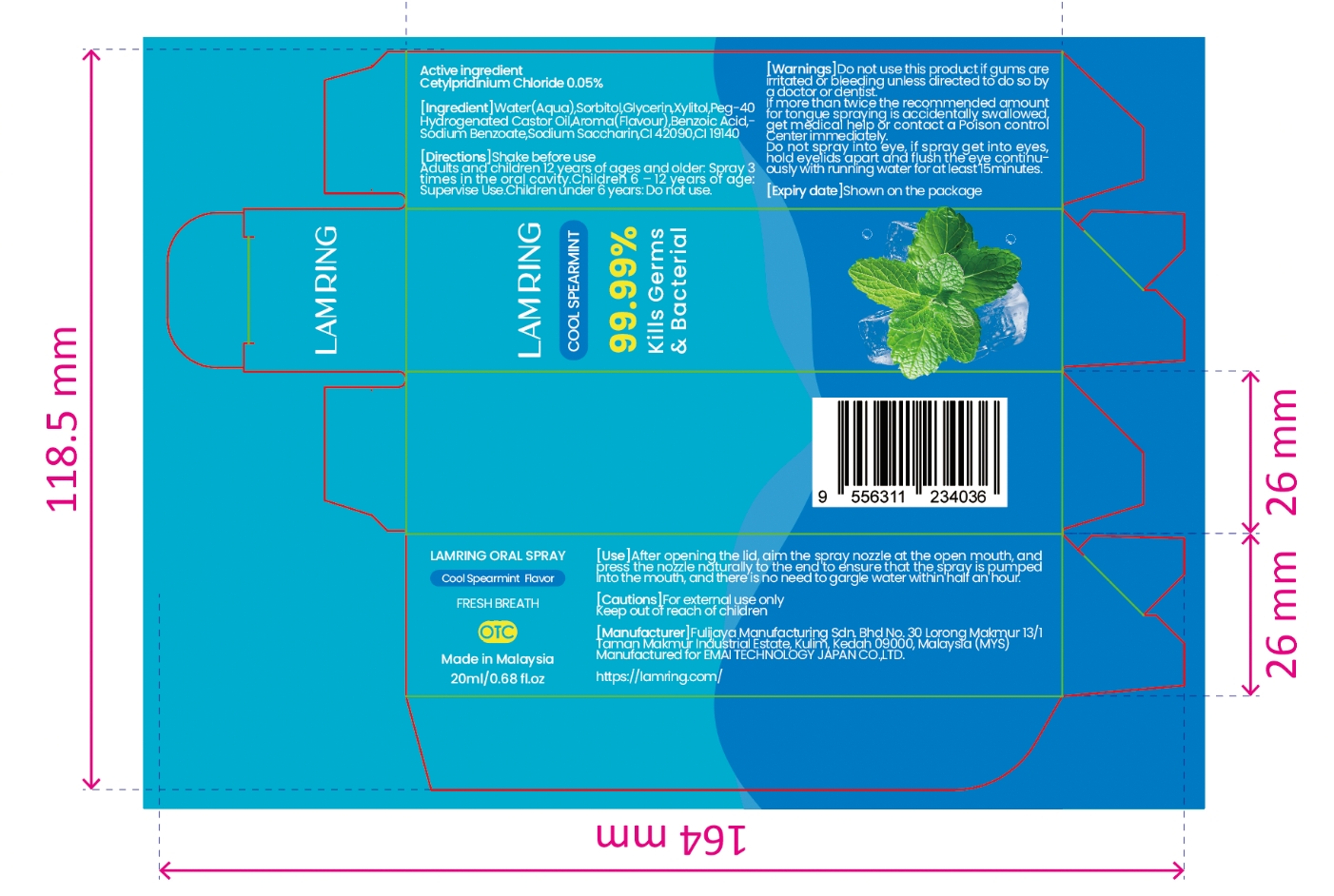
Active Ingredient(s)
Cetylpyridinium Chloride 0.05%
Purpose
Kills Germs & Bacterial
Use
After opening thelid, aim the spray nozzleat the open mouth, andpress thé nozzle naturallyto the end to ensure thatthe spray is pumped intothe mouth, and there isno need to gargle waterwithin half an hour.
Warnings
Do not use this product if gums areirritated or bleeding unless directed to do so bya doctor ordentistIfmore than twice the recommended amountfor tongue spraying is accidentally swallowedgetmedical help ör contact a Poison controlCenterimmediately.oodevare a one hesnixsersco era?Do not spray into '
Directions
Shake befor use. Adults and children 12 years of ages and older.Spray 3times in the oral cavity.Children 6-12years of age:Supervise use.Children under 6 years:Do not use
Caution
KEEP OUT OF REACH OF CHILDREN
Inactive ingredients
Water(Aqua),Sorbitol,Glycerin,Xylitol,Peg-40 Hydrogenated Castor Oil,Aroma(Flavour),BenzoicAcid,Sodium Benzoate,Sodium Saccharin,CI 14720
Package Label - Principal Display Panel