SODIUM BICARBONATE injection, solution
Sodium Bicarbonate by
Drug Labeling and Warnings
Sodium Bicarbonate by is a Prescription medication manufactured, distributed, or labeled by Phebra Pty Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HEALTH CARE PROVIDER LETTER
Important Prescribing Information
Subject: Temporary importation of 8.4% Sodium Bicarbonate Injection to address drug shortage issues
June 14, 2019
Dear Healthcare Professional,
Due to the current critical shortage of Sodium Bicarbonate Injection, USP in the United States (US) market, Athenex Pharmaceutical Division, LLC (Athenex) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of Sodium Bicarbonate Injection. Athenex has initiated temporary importation of another manufacturer's 8.4% Sodium Bicarbonate Injection (1 mEq/mL) into the U.S. market. This product is manufactured and marketed in Australia by Phebra Pty Ltd (Phebra).
At this time, no other entity except Athenex Pharmaceutical Division, LLC is authorized by the FDA to import or distribute Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, in the United States. FDA has not approved Phebra's 8.4% Sodium Bicarbonate Injection but does not object to its importation into the United States. Effective immediately, and during this temporary period, Athenex will offer the following presentation of Sodium Bicarbonate Injection:
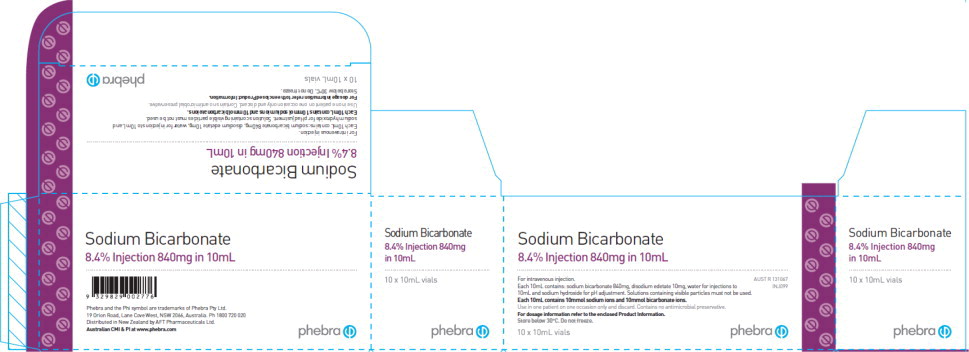
Sodium Bicarbonate Injection, 8.4% (1mEq/mL), 10mL per vial, 10 vials per carton
Ingredients: sodium bicarbonate, water for injection, disodium edetate and sodium hydroxide (pH adjustment)
Marketing Authorization Number in Australia is: 131067
Phebra's Sodium Bicarbonate Injection contains the same active ingredient, Sodium Bicarbonate, in the same strength and concentration, 8.4% (1 mEq/mL) as the U.S. registered Sodium Bicarbonate Injection, USP by Pfizer's subsidiary, Hospira. However, it is important to note that Phebra's Sodium Bicarbonate Injection (1 mEq/mL), is provided only in a Single Use 10 mL vials, whereas Hospira's product is provided in 50 mL single-dose vials and syringes. Any unused portion of Phebra's Sodium Bicarbonate Injection (1 mEq/mL) should be discarded after a single use.
There are some key differences in the labeling between the U.S. marketed Sodium Bicarbonate Injection and the imported product (please see the product comparison table at the end of this letter):
Sodium Bicarbonate Injection is only available by prescription in the U.S. Please refer to the FDA-approved package insert at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=077394 for the full prescribing information for 8.4% Sodium Bicarbonate Injection (1 mEq/mL).
The barcode may not register accurately on the U.S. scanning systems. Institutions should manually input the product into their systems and confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
To order or if you have questions about Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, please contact Athenex's Customer Service by phone at 1-855-273-0154.
To report adverse events or quality problems among patients who have received Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, please contact Athenex's Medical Affairs by phone at 1-855-273-0154. Adverse events or quality problems may also be reported to FDA's MedWatch Adverse Reporting Program either online, by regular mail or fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178)
If you have any questions about the information contained in this letter or the safe and effective use of Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, please contact Athenex 's Medical Affairs at 1-855-273-0154.
Sincerely,
Thomas J. Moutvic
Vice President, Regulatory Affairs
Athenex Pharmaceutical Division, LLCPfizer Phebra Molecular Formula NaHCO3 NaHCO3 Available Concentration 84 mg/mL and 75 mg/mL 840 mg/10 mL Route of Administration Intravenous Intravenous Unit of Use 8.4% and 7.5% in Ansyr II prefilled syringe, 50 mL vial 840 mg/10 mL (8.4%) sodium bicarbonate in water for injections
10 mL glass vial
Dosage Single-Dose Single use in one patient on one occasion only
pH 8.0 (7.0 to 8.5) 7.0 to 8.5
Claims Sterile, nonpyrogenic, hypertonic solution, system alkalizer, contain no bacteriostat, no antimicrobial agent or added buffer
Sterile solution, contains no antimicrobial agent Equivalency 84 mg equals 1 mEq of sodium and 1 mEq bicarbonate 84 mg equals 1 mEq of sodium (23 mg) and 1 mEq bicarbonate (61 mg)
Water for Injection, USP Water for Injections
Excipients for pH adjustment N/A disodium edetate and sodium hydroxide (for pH adjustment) Pharmacology (what is does) increases plasma bicarbonate buffers excess hydrogen ion concentration raises blood pH reverses clinical manifestations of acidosis systemic alkalinizing agent that:
increase plasma bicarbonate
buffer excess hydrogen ion concentration
raise blood pH
reverse clinical manifestations of acidosis
Pharmacology (in water) dissociates in water to provide sodium and bicarbonate ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Bicarbonate (HCO3–) is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mEq/liter.
dissociates in water to provide sodium and bicarbonate ions (HCO3–). Sodium is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Bicarbonate is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mmol/L. Pharmacology (kidney function) Plasma concentration is regulated by the kidney through acidification of the urine when there is a deficit or by alkalinization of the urine when there is an excess. Bicarbonate anion is considered "labile" since at a proper concentration of hydrogen ion (H+) it may be converted to carbonic acid (H2CO3) and thence to its volatile form, carbon dioxide (CO2) excreted by the lung. Normally a ratio of 1:20 (carbonic acid; bicarbonate) is present in the extracellular fluid. In a healthy adult with normal kidney function, practically all the glomerular filtered bicarbonate ion is reabsorbed; less than 1% is excreted in the urine. Acid-base homeostasis exerts a major influence on protein function, thereby critically affecting tissue and organ performance. Systemic arterial pH is maintained by extracellular and intracellular chemical buffering together with respiratory and renal regulatory mechnism. The control of arterial carbon dioxide (CO2) tension (PaCO2) by the central nervous system and respiratory systems and the control of the plasma bicarbonate by the kidneys stabilize the arterial pH by excretion or retention of acid or alkali. Under most circumstances, CO2 production and excretion are matched, and the usual steady-state PaCO2 is maintained at 40 mmHg. The kidneys regulate plasma HCO3– through three main processes: (1) "reabsorption" of filtered HCO3–, (2) formation of titratable acid, and (3) excretion of NH4+ in the urine. The kidney filters approximately 4000 mmol of HCO3– per day. To reabsorb the filtered load of HCO3–, the renal tubules must therefore secrete 4000 mmol of hydrogen ions. Between 80 and 90% of HCO3– is reabsorbed in the proximal tubule. The distal nephron reabsorbs the remainder and secretes protons, as generated from metabolism, to defend systemic pH. While this quantity of protons, 40 to 60 mmol/d, is small, it must be secreted to prevent chronic positive H+ balance and metabolic acidosis. This quantity of secreted protons is represented in the urine as titratable acid and NH4+. Metabolic acidosis in the face of normal renal function increases NH4+ production and excretion. NH4+ production and excretion are impaired in chronic renal failure, hyperkalaemia, and renal tubular acidosis.
The management of serious acid-base disorders always demands precise diagnosis and treatment of the underlying disease, and in certain circumstances, it requires steps to combat the deviation in systemic acidity itself. Administration of sodium bicarbonate will increase the plasma HCO3– concentration and help restore the plasma pH within the normal range (pH 7.35-7.45). Changes in acid-base balance also stimulate compensatory ion-exchange mechanisms. When the extracellular hydrogen ion concentration increases, as in acidosis, there is a redistribution of potassium ions from intracellular to extracellular fluid. Administration of sodium bicarbonate can cause a redistribution of potassium ions into cells in patients with acidosis, by increasing the plasma pH.
The urinary pH will be increased by sodium bicarbonate in patients with normal renal function. Alkalinising the urine can increase the solubility of certain weak acids, and can increase the ionisation and urinary excretion of lipid-soluble organic acids (e.g. phenobarbitone, salicylates).
Indications and Usage Sodium Bicarbonate Injection, USP is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest and severe primary lactic acidosis. Sodium bicarbonate is further indicated in the treatment of certain drug intoxications, including barbiturates (where dissociation of the barbiturate-protein complex is desired), in poisoning by salicylates or methyl alcohol and in hemolytic reactions requiring alkalinization of the urine to diminish nephrotoxicity of hemoglobin and its breakdown products. Sodium bicarbonate also is indicated in severe diarrhea which is often accompanied by a significant loss of bicarbonate. Treatment of metabolic acidosis should, if possible, be superimposed on measures designed to control the basic cause of the acidosis – e.g., insulin in uncomplicated diabetes, blood volume restoration in shock. But since an appreciable time interval may elapse before all of the ancillary effects are brought about, bicarbonate therapy is indicated to minimize risks inherent to the acidosis itself.
Vigorous bicarbonate therapy is required in any form of metabolic acidosis where a rapid increase in plasma total CO2 content is crucial – e.g., cardiac arrest, circulatory insufficiency due to shock or severe dehydration, and in severe primary lactic acidosis or severe diabetic acidosis.
Sodium Bicarbonate Injection is indicated as an alkalinising agent in the treatment of metabolic acidosis which may occur in many conditions including diabetes, starvation, hepatitis, cardiac arrest, shock, severe dehydration, renal insufficiency, severe diarrhoea, Addison's disease or administration of acidifying salts (e.g. excessive sodium chloride, calcium chloride, ammonium chloride).
Sodium Bicarbonate Injection is also used to increase urinary pH in order to increase the solubility of certain weak acids (e.g. cystine, sulphonamides, uric acid) and in the treatment of certain intoxications (e.g. methanol, phenobarbitone, salicylates, lithium) to decrease renal absorption of the drug or to correct acidosis.Contraindications Sodium Bicarbonate Injection, USP is contraindicated in patients who are losing chloride by vomiting or from continuous gastrointestinal suction, and in patients receiving diuretics known to produce a hypochloremic alkalosis. Sodium Bicarbonate Injection is contraindicated in patients with renal failure, respiratory or metabolic alkalosis, hypoventilation or chloride depletion, hypernatraemia, hypertension, oedema, congestive heart failure, eclampsia, aldosteronism, a history of urinary calculi and consistent potassium depletion or hypocalcaemia.
It is also generally contraindicated in patients with excessive chloride loss from vomiting or continuous gastrointestinal suctioning and in patients at risk of developing diuretic-induced hypochloraemic alkalosis.
Warnings and Precautions General
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
Extravascular infiltration should be avoided.
Do not use unless solution is clear and the container or seal is intact. Discard unused portion.
The potentially large loads of sodium given with bicarbonate require that caution be exercised in the use of sodium bicarbonate in patients with congestive heart failure or other edematous or sodium retaining states, as well as in patients with oliguria or anuria.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Potassium depletion may predispose to metabolic alkalosis and coexistent hypocalcemia may be associated with carpopedal spasm as the plasma pH rises. These dangers can be minimized if such electrolyte imbalances are appropriately treated prior to or concomitantly with bicarbonate infusion.Treatment strategies for metabolic acidosis are primarily directed towards the underlying cause. Bicarbonate therapy is a temporary measure used for severe acidosis.
Specialised texts and protocols should be consulted to guide use. Note that sodium bicarbonate 8.4% is a hypertonic solution.
Whenever respiratory acidosis is present with metabolic acidosis, both pulmonary ventilation and perfusion must be adequately supported to get rid of excess carbon dioxide.
Laboratory determination of the patient's acid-base status is recommended before and during treatment to minimise the possibility of overdosage and resultant metabolic alkalosis. Frequent monitoring of serum electrolyte concentrations is essential.
To minimise the risks of pre-existing hypokalaemia and/or hypocalcaemia, these electrolyte disturbances should be corrected prior to initiation of, or concomitantly with, sodium bicarbonate therapy.
Solutions containing sodium may cause fluid overload when given in excess, resulting in dilution of serum electrolytes, overhydration, congestive conditions or pulmonary oedema.
Excessively elevated plasma sodium concentrations may cause dehydration of the brain, resulting in somnolence and confusion, which may progress to convulsions, coma, respiratory failure and ultimately death.
Bicarbonate should be given with caution to patients with ‘type A' lactic acidosis (tissue hypoxia). Administration of bicarbonate will tend to limit the available oxygen, increase lactate production and thus worsen the acidosis.
Data from the literature are not in favour of the use of bicarbonate in the treatment of diabetic ketoacidosis with pH values between 6.90 and 7.10.
Sodium bicarbonate should be used with caution in patients with cirrhosis.
Accidental extravascular injection of hypertonic solutions may cause vascular irritation, chemical cellulitis (because of their alkalinity), subsequently resulting in tissue necrosis, ulceration and /or sloughing at the site of injection.
The use of scalp veins should be avoided.
Do not use the injection if it contains precipitate. Do not use unless the solution is clear and the container and seal are intact. Discard any unused portion.
Drug Interactions Additives may be incompatible; norepinephrine and dobutamine are incompatible with sodium bicarbonate solution.
The addition of sodium bicarbonate to parenteral solutions containing calcium should be avoided, except where compatibility has been previously established. Precipitation or haze may result from sodium bicarbonate/calcium admixtures. NOTE: Do not use the injection if it contains precipitate.
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.Alkalinisation of the urine leads to increased renal clearance of acidic drugs such as salicylates, tetracyclines, (especially doxycycline), barbiturates and tricyclic antidepressants. Conversely, it prolongs the half life and duration of basic drugs such as quinidine, amphetamines, ephedrine and pseudoephedrine and may result in toxicity.
Sodium bicarbonate enhances lithium excretion.
Solutions containing sodium ions should be used with great care, if at all, in patients receiving corticosteroids or corticotropin.
Hypochloraemic alkalosis may occur if sodium bicarbonate is used in conjunction with potassium depleting diuretics such as bumetanide, ethacrynic acid, frusemide and thiazides. Concurrent use in patients taking potassium supplements may reduce serum potassium concentration by promoting an intracellular ion shift.
The following drug may have enhanced or prolonged effects due to concomitant administration with sodium bicarbonate: flecainide.
The following drugs may have decreased effectiveness due to concomitant administration with sodium bicarbonate: aspirin and other salicylates, barbiturates and lithium.
The following drugs have been reported to be susceptible to inactivation on mixing with sodium bicarbonate solution: adrenaline HCl, benzylpenicillin potassium, carmustine, glycopyrrolate, isoprenaline HCl and suxamethonium chloride.
Compatibility / Incompatibility See above Sodium Bicarbonate Injection 8.4% can be diluted with 5% glucose injection or 0.9% sodium chloride injection. To reduce
microbiological hazard, use as soon as practicable after dilution. If storage is necessary, hold at 2°C-8°C for not more than 24 hours.
Sodium bicarbonate is incompatible with certain substances in solution and specialized literature should be consulted.
Incompatible Fluids / Medicines
Sodium bicarbonate is incompatible with acids, acidic salts and many alkaloidal salts. Sodium bicarbonate solutions should not be mixed with calcium or magnesium salts, cisplatin, dobutamine hydrochloride, labetalol hydrochloride or oxytetracycline hydrochloride as this may result in formation of insoluble precipitates. Sodium bicarbonate is also incompatible with corticotropin, hydromorphone hydrochloride, insulin, magnesium sulfate, methicillin sodium, narcotic salts, noradrenaline acid tartrate, pentobarbitone sodium, procaine hydrochloride, promazine hydrochloride (in glucose injection), streptomycin sulfate, tetracycline hydrochloride, thiopentone sodium, vancomycin hydrochloride, lactated Ringer's injection, sodium lactate injection or Ringer's injection.
The co-administration with other drugs is not recommended; medicines should not be added to, or run through the same giving set as sodium bicarbonate. Before the administration of other drugs, the cannula and intravenous tubing must be carefully irrigated with a 5 to 10 mL bolus of 0.9% sodium chloride injection following administration of sodium bicarbonate to avoid inactivation and precipitation.
The addition of sodium bicarbonate to solutions containing calcium should be avoided except where compatibility has been shown. Solutions turning hazy as a result of sodium bicarbonate/calcium admixtures should be discarded.
Laboratory Tests The aim of all bicarbonate therapy is to produce a substantial correction of the low total CO2 content and blood pH, but the risks of overdosage and alkalosis should be avoided. Hence, repeated fractional doses and periodic monitoring by appropriate laboratory tests are recommended to minimize the possibility of overdosage. False positive Labstix® for urine protein may result due to the high urinary alkalinity produced by sodium bicarbonate. Pregnancy Teratogenic Effects. Pregnancy Category C. Animal reproduction studies have not been conducted with sodium bicarbonate. It is also not known whether sodium bicarbonate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium bicarbonate should be given to a pregnant woman only if clearly needed. Use in Pregnancy and Lactation
Animal reproduction studies have not been conducted with sodium bicarbonate. Safety in pregnancy and lactation has not been established.
The use of Sodium Bicarbonate Injection, as with any drug, in pregnant or lactating women should only be undertaken if the expected benefit outweighs the possible risk to the mother and fetus or child.
Pediatric Pediatric
Rapid injection (10 mL/min) of hypertonic Sodium Bicarbonate Injection, USP solutions into neonates and children under two years of age may produce hypernatremia, a decrease in cerebrospinal fluid pressure and possible intracranial hemorrhage. The rate of administration in such patients should therefore be limited to no more than 8 mEq/kg/day. A 4.2% solution may be preferred for such slow administration. In emergencies such as cardiac arrest, the risk of rapid infusion must be weighed against the potential for fatality due to acidosis.Use in Children
Rapid injection (10 mL/min) of hypertonic Sodium Bicarbonate Injection solutions into neonates and children under 2 years of age may produce hypernatraemia, a decrease in cerebrospinal fluid pressure and possible intracranial haemorrhage. In emergency situations, such as cardiac arrest, the risk of rapid infusion of the drug must be weighed against the potential for death from acidosis. It should also be noted that administration of sodium bicarbonate to children undergoing cardiopulmonary resuscitation may worsen respiratory acidosis. Do not administer more than 8mmol/kg/day.
Geriatric Geriatric
Clinical studies of Sodium Bicarbonate Injection, USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
N/A Special Population (CHF and renal insufficiency) Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention. In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention. The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. Extravascular infiltration should be avoided The potentially large loads of sodium given with bicarbonate require that caution be exercised in the use of sodium bicarbonate in patients with congestive heart failure or other edematous or sodium retaining states, as well as in patients with oliguria or anuria. Use in patients with congestive heart failure or renal insufficiency
Sodium retention and oedema may occur during sodium bicarbonate therapy, especially when the drug is given in large doses or to patients with renal insufficiency, congestive heart failure or those predisposed to sodium retention and oedema. Sodium and water overload may result in hypernatraemia and hyperosmolality. Severe hyperosmolal states may develop during cardiopulmonary resuscitation when excessive doses of sodium bicarbonate are administered. Serum potassium may decrease during sodium bicarbonate therapy leading to hypokalaemia.
Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other oedematous or sodium-retaining conditions; in patients with renal insufficiency, especially those with severe insufficiency such as oliguria or anuria; and in patients receiving corticosteroids or corticotropin, since each gram of sodium bicarbonate contains 12mEq of sodium.
Adverse Reactions ADVERSE REACTIONS
Overly aggressive therapy with Sodium Bicarbonate Injection, USP can result in metabolic alkalosis (associated with muscular twitchings, irritability, and tetany) and hypernatremia.
Inadvertent extravasation of intravenously administered hypertonic solutions of sodium bicarbonate have been reported to cause chemical cellulitis because of their alkalinity, with tissue necrosis, ulceration or sloughing at the site of infiltration. Prompt elevation of the part, warmth and local injection of lidocaine or hyaluronidase are recommended to reduce the likelihood of tissue sloughing from extravasated I.V. solutions.ADVERSE EFFECTS
Metabolic alkalosis and/or hypokalaemia may ensue as a result of prolonged use or over correction of the bicarbonate deficit, especially in patients with impaired renal function. (see OVERDOSAGE)
Metabolic alkalosis may be accompanied by compensatory hyperventilation, paradoxical acidosis of the cerebrospinal fluid, severe hypokalaemia, hyperirritability or tetany.
Hypernatraemia has been reported with sodium bicarbonate use, especially in patients with renal disease.
Hyperosmolality has also been associated with sodium bicarbonate use.
Accidental extravasation of intravenous hypertonic solutions of sodium bicarbonate has been reported to cause chemical cellulitis, with tissue necrosis, tissue calcification, ulceration or sloughing at the site of infiltration. Prompt elevation of the part, warmth and local injection of lignocaine or hyaluronidase are recommended to prevent sloughing of extravasated intravenous infusions. Hyperirritability or tetany may occur, caused by rapid shifts of free ionised calcium or due to serum protein alterations arising from the pH changes.
Cerebral oedema has occurred with sodium bicarbonate use and a possibility of intracranial haemorrhage exists.
Hypercapnia has occurred in patients receiving sodium bicarbonate and with fixed ventilation.
Overdosage Overdosage
Should alkalosis result, the bicarbonate should be stopped and the patient managed according to the degree of alkalosis present. 0.9% sodium chloride injection intravenous may be given; potassium chloride also may be indicated if there is hypokalemia. Severe alkalosis may be accompanied by hyperirritability or tetany and these symptoms may be controlled by calcium gluconate. An acidifying agent such as ammonium chloride may also be indicated in severe alkalosis.OVERDOSAGE
Alkalosis is a result of overdosage.
Symptoms of Overdosage
Symptoms include mood changes, tiredness, slow breathing, muscle weakness and irregular heartbeat. Muscle hypertonicity, twitching and tetany may develop, especially in hypocalcaemic patients.
Metabolic alkalosis, which may be accompanied by compensatory hyperventilation, paradoxical acidosis of the cerebrospinal fluid, severe hypokalaemia, hyperirritability or tetany.
Treatment of Overdosage
Treatment of metabolic alkalosis associated with bicarbonate overdose consists mainly of appropriate correction of fluid and electrolyte balance. Replacement of calcium, chloride and potassium ions may be of particular importance.
The bicarbonate should be stopped and the patient managed according to the degree of alkalosis present. To control the symptoms of alkalosis the patient should rebreathe expired air. Sodium chloride injection 0.9% may be given intravenously; potassium chloride also may be indicated if there is hypokalaemia.
Calcium gluconate may be used to control hyperirritability and tetany which can occur in severe alkalosis. Ammonium chloride may also be indicated as an acidifying agent in severe cases (except in patients with pre-existing hepatic disease).
Treatment of hypernatraemia usually requires water replacement; restricted sodium intake and oral water may be sufficient. If more severe, glucose 5% may be administered by slow intravenous infusion. If total body sodium is too high, loop diuretics combined with an infusion of glucose 5% and potassium supplementation may be necessary.
DOSAGE AND ADMINISTRATION DOSAGE AND ADMINISTRATION
Sodium Bicarbonate Injection, USP is administered by the intravenous route.
In cardiac arrest, a rapid intravenous dose of one to two 50 mL syringes (44.6 to 100 mEq) may be given initially and continued at a rate of 50 mL (44.6 to 50 mEq) every 5 to 10 minutes if necessary (as indicated by arterial pH and blood gas monitoring) to reverse the acidosis. Caution should be observed in emergencies where very rapid infusion of large quantities of bicarbonate is indicated. Bicarbonate solutions are hypertonic and may produce an undesirable rise in plasma sodium concentration in the process of correcting the metabolic acidosis. In cardiac arrest, however, the risks from acidosis exceed those of hypernatremia.DOSAGE AND ADMINISTRATION
Dosage of Sodium Bicarbonate Injection is determined by the severity of the acidosis, appropriate laboratory determinations, and the patient's age, weight and clinical condition.
Sodium Bicarbonate Injection is administered by the intravenous route preferably via a central line. Extravasation must be avoided; the solution is hypertonic and irritant to veins resulting in extensive skin necrosis if the solution leaks from the vein in the tissues. Intramuscular injection is not recommended.
Contains no antimicrobial agent and is for single use in one patient on one occasion only.
Cardiac Arrest or Severe Metabolic Acidosis - Administration is based on the results of arterial blood pH, PaCO2 and calculation of base deficit.
In cardiac arrest, an initial direct intravenous dose of 1 mmol/kg (1 mL/kg of an 8.4% sodium bicarbonate solution) may be given, followed by 0.5 mmol/kg (0.5 mL/kg of an 8.4% sodium bicarbonate solution) at ten minute intervals depending on arterial blood gases and according to the appropriate treatment protocol and guidelines.
Adequate alveolar ventilation should be ensured during cardiac arrest and administration of sodium bicarbonate, since adequate ventilation contributes to the correction of acidosis and since administration of sodium bicarbonate is followed by release of carbon dioxide.
Children – The usual dose is 1 mmol/kg (1mL/kg of an 8.4% sodium bicarbonate injection) given by slow intravenous injection.
Infants (up to 2 years of age) - In infants (up to 2 years of age) the solution should be diluted with an equal amount (1:1 ratio) of 5% glucose or water for injections (to make 4.2% sodium bicarbonate solution) for slow intravenous administration and at a dose not to exceed 8mmol/kg/day, and according to the appropriate treatment protocol and guidelines. This diluted solution is hypertonic. Slow administration rates and a 4.2% solution are recommended in neonates to minimise the possibility of producing hypernatraemia, decreasing cerebrospinal fluid pressure and inducing intracranial haemorrhage. (See PRECAUTIONS and ADVERSE EFFECTS)
Sodium bicarbonate should only be given if the child is being effectively ventilated as any carbon dioxide that is released by the process of acid neutralisation must be removed from the body via the lungs or paradoxical intracellular acidosis will result.
DOSAGE AND ADMINISTRATION (continued) In less urgent forms of metabolic acidosis, Sodium Bicarbonate Injection, USP may be added to other intravenous fluids. The amount of bicarbonate to be given to older children and adults over a four-to-eight-hour period is approximately 2 to 5 mEq/kg of body weight – depending upon the severity of the acidosis as judged by the lowering of total CO2 content, blood pH and clinical condition of the patient. In metabolic acidosis associated with shock, therapy should be monitored by measuring blood gases, plasma osmolarity, arterial blood lactate, hemodynamics and cardiac rhythm. Bicarbonate therapy should always be planned in a stepwise fashion since the degree of response from a given dose is not precisely predictable. Initially an infusion of 2 to 5 mEq/kg body weight over a period of 4 to 8 hours will produce a measurable improvement in the abnormal acid-base status of the blood. The next step of therapy is dependent upon the clinical response of the patient. If severe symptoms have abated, then the frequency of administration and the size of the dose may be reduced.
In general, it is unwise to attempt full correction of a low total CO2 content during the first 24 hours of therapy, since this may be accompanied by an unrecognized alkalosis because of a delay in the readjustment of ventilation to normal. Owing to this lag, the achievement of total CO2 content of about 20 mEq/liter at the end of the first day of therapy will usually be associated with a normal blood pH. Further modification of the acidosis to completely normal values usually occurs in the presence of normal kidney function when and if the cause of the acidosis can be controlled. Values for total CO2 which are brought to normal or above normal within the first day of therapy are very likely to be associated with grossly alkaline values for blood pH, with ensuing undesired side effects.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Intravenous Infusion- In less urgent forms of metabolic acidosis, Sodium Bicarbonate Injection may be added to 5% glucose for intravenous infusion. (See COMPATIBILITY / INCOMPATIBILITY)
Sodium Bicarbonate 8.4% Injection can be diluted with 5% glucose injection or 0.9% sodium chloride injection. To reduce microbiological hazard, use as soon as practicable after dilution. If storage is necessary, hold at 2°C-8°C for not more than 24 hours.
Sodium Bicarbonate Injection for intravenous infusion is preferably administered in a large vein, over 4 to 8 hours in mild conditions of metabolic acidosis.
The amount of bicarbonate to be given as intravenous infusion to older children and adults over a 4 to 8 hour period is approximately 2 to 5 mmol/kg of bodyweight, depending upon the severity of the acidosis as judged by the lowering of the total CO2 content, blood pH and clinical condition of the patient. Standard texts and institutional protocols specific to the underlying disorder should be consulted for calculation of individual dosage.
Bicarbonate therapy should always be planned in a stepwise fashion since the degree of response from a given dose is not precisely predictable.
In general, it is unwise to attempt full correction of a low total CO2 content during the first 24 hours of therapy, since this may be accompanied by an unrecognised alkalosis because of a delay in the readjustment of ventilation to normal.Storage Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Store below 30°C. Do not freeze. - SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Sodium Bicarbonate Injection is a sterile solution containing 84 mg/mL sodium bicarbonate in water for injections. The excipients are disodium edetate, sodium hydroxide (for pH adjustment) and water for injections. The pH is approximately 7.0-8.5.
Each mL of solution contains 84.0 mg of sodium bicarbonate which gives 23.0 mg (or 1 mmol or 1 mEq) of sodium and 61.0 mg (or 1 mmol or 1 mEq) of bicarbonate.
-
PHARMACOLOGY
Sodium bicarbonate is a systemic alkalinizing agent which, when given intravenously, will increase plasma bicarbonate, buffer excess hydrogen ion concentration, raise blood pH and reverse the clinical manifestations of acidosis.
Sodium bicarbonate dissociates in water to provide sodium and bicarbonate ions (HCO3–). Sodium is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Bicarbonate is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mmol/L.
Acid-base homeostasis exerts a major influence on protein function, thereby critically affecting tissue and organ performance. Systemic arterial pH is maintained by extracellular and intracellular chemical buffering together with respiratory and renal regulatory mechnism. The control of arterial carbon dioxide (CO2) tension (PaCO2) by the central nervous system and respiratory systems and the control of the plasma bicarbonate by the kidneys stabilize the arterial pH by excretion or retention of acid or alkali. Under most circumstances, CO2 production and excretion are matched, and the usual steady-state PaCO2 is maintained at 40 mmHg. The kidneys regulate plasma HCO3– through three main processes: (1) "reabsorption" of filtered HCO3–, (2) formation of titratable acid, and (3) excretion of NH4+ in the urine. The kidney filters approximately 4000 mmol of HCO3– per day. To reabsorb the filtered load of HCO3–, the renal tubules must therefore secrete 4000 mmol of hydrogen ions. Between 80 and 90% of HCO3– is reabsorbed in the proximal tubule. The distal nephron reabsorbs the remainder and secretes protons, as generated from metabolism, to defend systemic pH. While this quantity of protons, 40 to 60 mmol/d, is small, it must be secreted to prevent chronic positive H+ balance and metabolic acidosis. This quantity of secreted protons is represented in the urine as titratable acid and NH4+. Metabolic acidosis in the face of normal renal function increases NH4+ production and excretion. NH4+ production and excretion are impaired in chronic renal failure, hyperkalaemia, and renal tubular acidosis.
The management of serious acid-base disorders always demands precise diagnosis and treatment of the underlying disease, and in certain circumstances, it requires steps to combat the deviation in systemic acidity itself. Administration of sodium bicarbonate will increase the plasma HCO3– concentration and help restore the plasma pH within the normal range (pH 7.35-7.45). Changes in acid-base balance also stimulate compensatory ion-exchange mechanisms. When the extracellular hydrogen ion concentration increases, as in acidosis, there is a redistribution of potassium ions from intracellular to extracellular fluid. Administration of sodium bicarbonate can cause a redistribution of potassium ions into cells in patients with acidosis, by increasing the plasma pH.
The urinary pH will be increased by sodium bicarbonate in patients with normal renal function. Alkalinising the urine can increase the solubility of certain weak acids, and can increase the ionisation and urinary excretion of lipid-soluble organic acids (e.g. phenobarbitone, salicylates).
Metabolic Acidosis
Metabolic acidosis is characterized by a primary decrease in serum bicarbonate (HCO3–) concentration to below 24 mmol/L and a compensatory decrease in the CO2 concentration. It occurs from either loss of bicarbonate (HCO3–) or addition of hydrogen ion (H+). Bicarbonate loss generally occurs through the kidneys or the bowel. Acidosis from decreased acid renal excretion generally is slow to develop. In contrast, acidosis from increased acid production, as in lactic acidosis or ketoacidosis, can exceed maximal renal excretion and cause a rapidly developing, severe acidosis.
Standard texts and institutional protocols should be consulted on the aetiology and management of metabolic acidosis.
-
INDICATIONS
Sodium Bicarbonate Injection is indicated as an alkalinising agent in the treatment of metabolic acidosis which may occur in many conditions including diabetes, starvation, hepatitis, cardiac arrest, shock, severe dehydration, renal insufficiency, severe diarrhoea, Addison's disease or administration of acidifying salts (e.g. excessive sodium chloride, calcium chloride, ammonium chloride).
Sodium Bicarbonate Injection is also used to increase urinary pH in order to increase the solubility of certain weak acids (e.g. cystine, sulphonamides, uric acid) and in the treatment of certain intoxications (e.g. methanol, phenobarbitone, salicylates, lithium) to decrease renal absorption of the drug or to correct acidosis.
-
CONTRAINDICATIONS
Sodium Bicarbonate Injection is contraindicated in patients with renal failure, respiratory or metabolic alkalosis, hypoventilation or chloride depletion, hypernatraemia, hypertension, oedema, congestive heart failure, eclampsia, aldosteronism, a history of urinary calculi and consistent potassium depletion or hypocalcaemia.
It is also generally contraindicated in patients with excessive chloride loss from vomiting or continuous gastrointestinal suctioning and in patients at risk of developing diuretic-induced hypochloraemic alkalosis.
-
PRECAUTIONS
Treatment strategies for metabolic acidosis are primarily directed towards the underlying cause. Bicarbonate therapy is a temporary measure used for severe acidosis.
Specialised texts and protocols should be consulted to guide use. Note that sodium bicarbonate 8.4% is a hypertonic solution.
Whenever respiratory acidosis is present with metabolic acidosis, both pulmonary ventilation and perfusion must be adequately supported to get rid of excess carbon dioxide.
Laboratory determination of the patient's acid-base status is recommended before and during treatment to minimise the possibility of overdosage and resultant metabolic alkalosis. Frequent monitoring of serum electrolyte concentrations is essential.
To minimise the risks of pre-existing hypokalaemia and/or hypocalcaemia, these electrolyte disturbances should be corrected prior to initiation of, or concomitantly with, sodium bicarbonate therapy.
Solutions containing sodium may cause fluid overload when given in excess, resulting in dilution of serum electrolytes, overhydration, congestive conditions or pulmonary oedema.
Excessively elevated plasma sodium concentrations may cause dehydration of the brain, resulting in somnolence and confusion, which may progress to convulsions, coma, respiratory failure and ultimately death.
Bicarbonate should be given with caution to patients with ‘type A’ lactic acidosis (tissue hypoxia). Administration of bicarbonate will tend to limit the available oxygen, increase lactate production and thus worsen the acidosis.
Data from the literature are not in favour of the use of bicarbonate in the treatment of diabetic ketoacidosis with pH values between 6.90 and 7.10.
Sodium bicarbonate should be used with caution in patients with cirrhosis.
Accidental extravascular injection of hypertonic solutions may cause vascular irritation, chemical cellulitis (because of their alkalinity), subsequently resulting in tissue necrosis, ulceration and /or sloughing at the site of injection.
The use of scalp veins should be avoided.
Do not use the injection if it contains precipitate. Do not use unless the solution is clear and the container and seal are intact. Discard any unused portion.
Use in patients with congestive heart failure or renal insufficiency
Sodium retention and oedema may occur during sodium bicarbonate therapy, especially when the drug is given in large doses or to patients with renal insufficiency, congestive heart failure or those predisposed to sodium retention and oedema. Sodium and water overload may result in hypernatraemia and hyperosmolality. Severe hyperosmolal states may develop during cardiopulmonary resuscitation when excessive doses of sodium bicarbonate are administered. Serum potassium may decrease during sodium bicarbonate therapy leading to hypokalaemia.
Sodium bicarbonate should be used with extreme caution in patients with congestive heart failure or other oedematous or sodium-retaining conditions; in patients with renal insufficiency, especially those with severe insufficiency such as oliguria or anuria; and in patients receiving corticosteroids or corticotropin, since each gram of sodium bicarbonate contains 12mEq of sodium.
Use in Children
Rapid injection (10 mL/min) of hypertonic Sodium Bicarbonate Injection solutions into neonates and children under 2 years of age may produce hypernatraemia, a decrease in cerebrospinal fluid pressure and possible intracranial haemorrhage. In emergency situations, such as cardiac arrest, the risk of rapid infusion of the drug must be weighed against the potential for death from acidosis. It should also be noted that administration of sodium bicarbonate to children undergoing cardiopulmonary resuscitation may worsen respiratory acidosis. Do not administer more than 8mmol/kg/day (See DOSAGE AND ADMINISTRATION)
Use in Pregnancy and Lactation
Animal reproduction studies have not been conducted with sodium bicarbonate. Safety in pregnancy and lactation has not been established.
The use of Sodium Bicarbonate Injection, as with any drug, in pregnant or lactating women should only be undertaken if the expected benefit outweighs the possible risk to the mother and fetus or child.
-
INTERACTIONS WITH OTHER MEDICINES
Alkalinisation of the urine leads to increased renal clearance of acidic drugs such as salicylates, tetracyclines, (especially doxycycline), barbiturates and tricyclic antidepressants. Conversely, it prolongs the half life and duration of basic drugs such as quinidine, amphetamines, ephedrine and pseudoephedrine and may result in toxicity.
Sodium bicarbonate enhances lithium excretion.
Solutions containing sodium ions should be used with great care, if at all, in patients receiving corticosteroids or corticotropin.
Hypochloraemic alkalosis may occur if sodium bicarbonate is used in conjunction with potassium depleting diuretics such as bumetanide, ethacrynic acid, frusemide and thiazides. Concurrent use in patients taking potassium supplements may reduce serum potassium concentration by promoting an intracellular ion shift.
The following drug may have enhanced or prolonged effects due to concomitant administration with sodium bicarbonate: flecainide.
The following drugs may have decreased effectiveness due to concomitant administration with sodium bicarbonate: aspirin and other salicylates, barbiturates and lithium.
The following drugs have been reported to be susceptible to inactivation on mixing with sodium bicarbonate solution: adrenaline HCl, benzylpenicillin potassium, carmustine, glycopyrrolate, isoprenaline HCl and suxamethonium chloride.
-
COMPATIBILITY / INCOMPATIBILITY
Sodium Bicarbonate Injection 8.4% can be diluted with 5% glucose injection or 0.9% sodium chloride injection. To reduce microbiological hazard, use as soon as practicable after dilution. If storage is necessary, hold at 2°C-8°C for not more than 24 hours. (See DOSAGE AND ADMINISTRATION)
Sodium bicarbonate is incompatible with certain substances in solution and specialised literature should be consulted.
Incompatible Fluids / Medicines
Sodium bicarbonate is incompatible with acids, acidic salts and many alkaloidal salts. Sodium bicarbonate solutions should not be mixed with calcium or magnesium salts, cisplatin, dobutamine hydrochloride, labetalol hydrochloride or oxytetracycline hydrochloride as this may result in formation of insoluble precipitates. Sodium bicarbonate is also incompatible with corticotropin, hydromorphone hydrochloride, insulin, magnesium sulfate, methicillin sodium, narcotic salts, noradrenaline acid tartrate, pentobarbitone sodium, procaine hydrochloride, promazine hydrochloride (in glucose injection), streptomycin sulfate, tetracycline hydrochloride, thiopentone sodium, vancomycin hydrochloride, lactated Ringer's injection, sodium lactate injection or Ringer's injection.
The co-administration with other drugs is not recommended; medicines should not be added to, or run through the same giving set as sodium bicarbonate. Before the administration of other drugs, the cannula and intravenous tubing must be carefully irrigated with a 5 to 10 mL bolus of 0.9% sodium chloride injection following administration of sodium bicarbonate to avoid inactivation and precipitation.
The addition of sodium bicarbonate to solutions containing calcium should be avoided except where compatibility has been shown. Solutions turning hazy as a result of sodium bicarbonate/calcium admixtures should be discarded.
Effects on Laboratory Tests
False positive Labstix® for urine protein may result due to the high urinary alkalinity produced by sodium bicarbonate.
-
ADVERSE EFFECTS
Metabolic alkalosis and/or hypokalaemia may ensue as a result of prolonged use or over correction of the bicarbonate deficit, especially in patients with impaired renal function. (see OVERDOSAGE)
Metabolic alkalosis may be accompanied by compensatory hyperventilation, paradoxical acidosis of the cerebrospinal fluid, severe hypokalaemia, hyperirritability or tetany.
Hypernatraemia has been reported with sodium bicarbonate use, especially in patients with renal disease.
Hyperosmolality has also been associated with sodium bicarbonate use.
Accidental extravasation of intravenous hypertonic solutions of sodium bicarbonate has been reported to cause chemical cellulitis, with tissue necrosis, tissue calcification, ulceration or sloughing at the site of infiltration. Prompt elevation of the part, warmth and local injection of lignocaine or hyaluronidase are recommended to prevent sloughing of extravasated intravenous infusions. Hyperirritability or tetany may occur, caused by rapid shifts of free ionised calcium or due to serum protein alterations arising from the pH changes.
Cerebral oedema has occurred with sodium bicarbonate use and a possibility of intracranial haemorrhage exists.
Hypercapnia has occurred in patients receiving sodium bicarbonate and with fixed ventilation.
-
DOSAGE AND ADMINISTRATION
Dosage of Sodium Bicarbonate Injection is determined by the severity of the acidosis, appropriate laboratory determinations, and the patient's age, weight and clinical condition.
Sodium Bicarbonate Injection is administered by the intravenous route preferably via a central line. Extravasation must be avoided; the solution is hypertonic and irritant to veins resulting in extensive skin necrosis if the solution leaks from the vein in the tissues. Intramuscular injection is not recommended.
Contains no antimicrobial agent and is for single use in one patient on one occasion only.
Cardiac Arrest or Severe Metabolic Acidosis - Administration is based on the results of arterial blood pH, PaCO2 and calculation of base deficit.
In cardiac arrest, an initial direct intravenous dose of 1 mmol/kg (1 mL/kg of an 8.4% sodium bicarbonate solution) may be given, followed by 0.5 mmol/kg (0.5 mL/kg of an 8.4% sodium bicarbonate solution) at ten minute intervals depending on arterial blood gases and according to the appropriate treatment protocol and guidelines.
Adequate alveolar ventilation should be ensured during cardiac arrest and administration of sodium bicarbonate, since adequate ventilation contributes to the correction of acidosis and since administration of sodium bicarbonate is followed by release of carbon dioxide.
Children – The usual dose is 1 mmol/kg (1mL/kg of an 8.4% sodium bicarbonate injection) given by slow intravenous injection.
Infants (up to 2 years of age) - In infants (up to 2 years of age) the solution should be diluted with an equal amount (1:1 ratio) of 5% glucose or water for injections (to make 4.2% sodium bicarbonate solution) for slow intravenous administration and at a dose not to exceed 8mmol/kg/day, and according to the appropriate treatment protocol and guidelines. This diluted solution is hypertonic. Slow administration rates and a 4.2% solution are recommended in neonates to minimise the possibility of producing hypernatraemia, decreasing cerebrospinal fluid pressure and inducing intracranial haemorrhage. (See PRECAUTIONS and ADVERSE EFFECTS)
Sodium bicarbonate should only be given if the child is being effectively ventilated as any carbon dioxide that is released by the process of acid neutralisation must be removed from the body via the lungs or paradoxical intracellular acidosis will result.
Intravenous Infusion- In less urgent forms of metabolic acidosis, Sodium Bicarbonate Injection may be added to 5% glucose for intravenous infusion. (See COMPATIBILITY / INCOMPATIBILITY)
Sodium Bicarbonate 8.4% Injection can be diluted with 5% glucose injection or 0.9% sodium chloride injection. To reduce microbiological hazard, use as soon as practicable after dilution. If storage is necessary, hold at 2°C-8°C for not more than 24 hours.
Sodium Bicarbonate Injection for intravenous infusion is preferably administered in a large vein, over 4 to 8 hours in mild conditions of metabolic acidosis.
The amount of bicarbonate to be given as intravenous infusion to older children and adults over a 4 to 8 hour period is approximately 2 to 5 mmol/kg of bodyweight, depending upon the severity of the acidosis as judged by the lowering of the total CO2 content, blood pH and clinical condition of the patient. Standard texts and institutional protocols specific to the underlying disorder should be consulted for calculation of individual dosage.
Bicarbonate therapy should always be planned in a stepwise fashion since the degree of response from a given dose is not precisely predictable.
In general, it is unwise to attempt full correction of a low total CO2 content during the first 24 hours of therapy, since this may be accompanied by an unrecognised alkalosis because of a delay in the readjustment of ventilation to normal.
-
OVERDOSAGE
Alkalosis is a result of overdosage.
Symptoms of Overdosage
Symptoms include mood changes, tiredness, slow breathing, muscle weakness and irregular heartbeat. Muscle hypertonicity, twitching and tetany may develop, especially in hypocalcaemic patients.
Metabolic alkalosis, which may be accompanied by compensatory hyperventilation, paradoxical acidosis of the cerebrospinal fluid, severe hypokalaemia, hyperirritability or tetany.
Treatment of Overdosage
Treatment of metabolic alkalosis associated with bicarbonate overdose consists mainly of appropriate correction of fluid and electrolyte balance. Replacement of calcium, chloride and potassium ions may be of particular importance.
The bicarbonate should be stopped and the patient managed according to the degree of alkalosis present. To control the symptoms of alkalosis the patient should rebreathe expired air. Sodium chloride injection 0.9% may be given intravenously; potassium chloride also may be indicated if there is hypokalaemia.
Calcium gluconate may be used to control hyperirritability and tetany which can occur in severe alkalosis. Ammonium chloride may also be indicated as an acidifying agent in severe cases (except in patients with pre-existing hepatic disease).
Treatment of hypernatraemia usually requires water replacement; restricted sodium intake and oral water may be sufficient. If more severe, glucose 5% may be administered by slow intravenous infusion. If total body sodium is too high, loop diuretics combined with an infusion of glucose 5% and potassium supplementation may be necessary.
-
PRESENTATION AND STORAGE CONDITIONS
Sodium Bicarbonate Injection contains 840 mg/10 mL (8.4%) sodium bicarbonate in water for injections. It also contains disodium edetate and sodium hydroxide (for pH adjustment).
It is presented in a 10 mL glass vial as a pack of 10.
AUST R 131067
Phebra product code- INJ099
Store below 30°C. Do not freeze.
NAME AND ADDRESS OF THE SPONSOR
Phebra Pty Ltd, 19 Orion Road, Lane Cove West, NSW 2066, Australia Telephone: 1800 720 020
Distributed in New Zealand: AFT Pharmaceuticals Ltd, P O Box 33-203 Takapuna Auckland
POISON SCHEDULE OF THE MEDICINE
Unscheduled
Date of first inclusion in the ARTG: 2 February 2007
Date of most recent amendment: 18 September 2013
Phebra and the Phi symbol are trademarks of Phebra Pty Ltd, 19 Orion Road, Lane Cove West, NSW 2066, Australia.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SODIUM BICARBONATE
sodium bicarbonate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71456-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium bicarbonate (UNII: 8MDF5V39QO) (bicarbonate ion - UNII:HN1ZRA3Q20) sodium bicarbonate 84 mg in 1 mL Inactive Ingredients Ingredient Name Strength edetate disodium (UNII: 7FLD91C86K) sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71456-001-01 10 in 1 CARTON 05/24/2017 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 05/24/2017 Labeler - Phebra Pty Ltd (756928503)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.