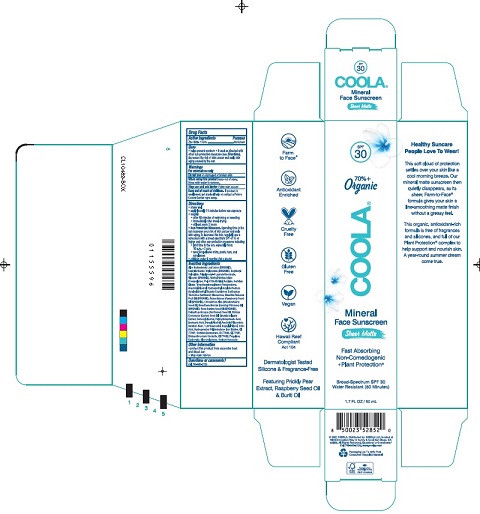
COOLA Mineral Face Sunscreen Sheer Matte SPF 30
Sheer Matte SPF 30 by
Drug Labeling and Warnings
Sheer Matte SPF 30 by is a Otc medication manufactured, distributed, or labeled by COOLA, Bentley Laboratories, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SHEER MATTE SPF 30- zinc oxide 10.5% lotion
COOLA
----------
COOLA Mineral Face Sunscreen Sheer Matte SPF 30
Uses helps prevent sunburn if used as directed with other
sun protection measures(see
Directions) decreases the risk of
skin cancer and early skin aging caused by the sun
Keep out of reach of children.If product is swallowed
get medical help or contact a Poison Control Center right away.
Directions
shake well
apply liberally 15 minutes before sun exposure
reapply:
after 80 minutes swimming or sweating
immediately after towel drying
at least every 2 hours
Sun Protection Measures.Spending time in the
sun increases your risk of skin cancer and early
skin aging. To decrease this risk, regularly use a
sunscreen with a broad spectrum SPF of 15 or
higher and after sun protection measures including:
limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeve shirts, pants, hats, and sunglasses
children under 6 months: Ask a doctor
Inactive Ingredients
Aloe Barbadensis Leaf Juice (ORGANIC),
Caprylic/Capric Triglyceride (ORGANIC), Butyloctyl
Salicylate, Polyglyceryl-4 Laurate/Succinate,
Glycerin (ORGANIC), Methyl Methacrylate
Crosspolymer, Poly C-10-30 Alkyl Acrylate, Sorbitan
Oleate, Trimethoxybenzylidene Pentanedione,
Arachidyl Alcohol, Hydroxyethyl Acrylate/Sodium
Acryloyldimethyl Taurate Copolymer, Carthamus
Tinctorius (Safflower) Oleosomes, Mauritia Flexuosa
Fruit Oil (ORGANIC), Rubus Idaeus (Raspberry) Seed
Oil (ORGANIC), Limnanthes Alba (Meadowfoam)
Seed Oil, Oenothera Biennis (Evening Primrose) Oil
(ORGANIC), Rosa Canina Seed Oil (ORGANIC),
Helianthus Annuus (Sunflower) Seed Oil, Ricinus
Communis (Castor) Seed Oil, Opuntia Vulgaris
Extract, Behenyl Alcohol, Polyhydroxystearic Acid,
Isostearic Acid, Benzyl Alcohol, Arachidyl Glucoside,
Xanthan Gum, 1,2-Hexanediol, Caprylyl Glycol, Citric
Acid, Hydrogenated Polyisobutene, Iron Oxides, Cl
77891, Sorbitan Isostearate, Cl 77492, Cl 77491,
Disteardimonium Hectorite, Cl 77499, Propylene
Carbonate, Gluconolactone, Sodium Benzoate
| SHEER MATTE SPF 30
zinc oxide 10.5% lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - COOLA (956990290) |
| Registrant - Bentley Laboratories, LLC (068351753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bentley Laboratories, LLC | 068351753 | manufacture(79753-044) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.