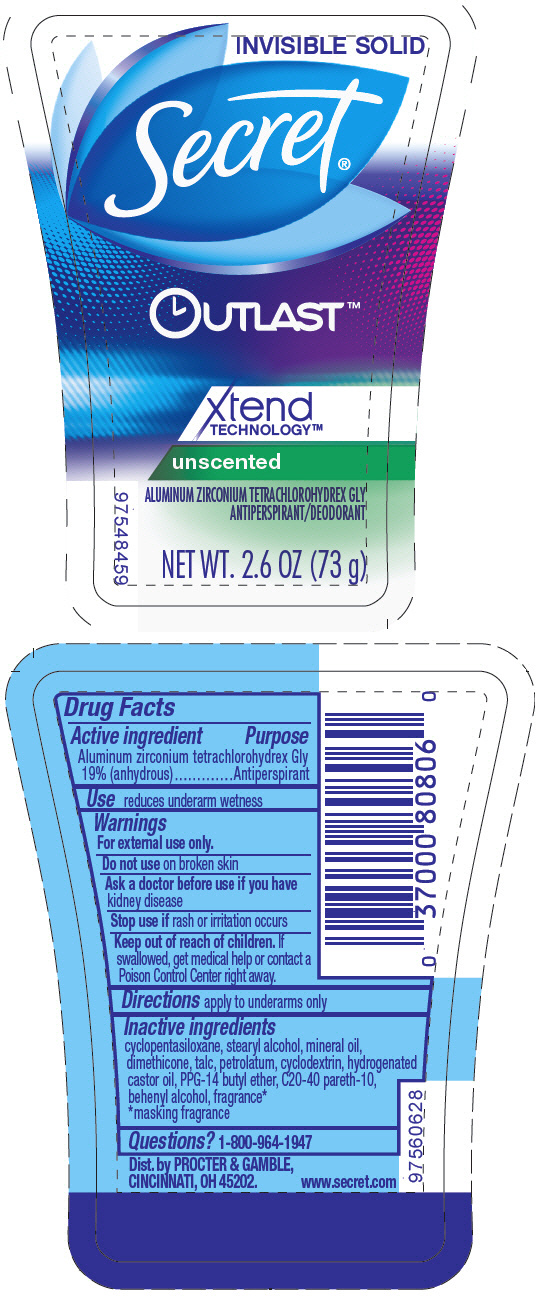
SECRET OUTLAST XTEND TECHNOLOGY INVISIBLE UNSCENTED- aluminum zirconium tetrachlorohydrex gly stick
Secret Outlast Xtend Technology Invisible by
Drug Labeling and Warnings
Secret Outlast Xtend Technology Invisible by is a Otc medication manufactured, distributed, or labeled by Procter and Gamble Manufacturing Company, Procter and Gamble Manufacturing Co.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 73 g Cylinder Label
-
INGREDIENTS AND APPEARANCE
SECRET OUTLAST XTEND TECHNOLOGY INVISIBLE UNSCENTED
aluminum zirconium tetrachlorohydrex gly stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69423-084 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY (UNII: 8O386558JE) (ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY - UNII:8O386558JE) ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY 19 g in 100 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) MINERAL OIL (UNII: T5L8T28FGP) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) PETROLATUM (UNII: 4T6H12BN9U) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) PPG-14 BUTYL ETHER (UNII: R199TJT95T) C20-40 PARETH-10 (UNII: TE3MZI4V3F) DOCOSANOL (UNII: 9G1OE216XY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69423-084-73 73 g in 1 CYLINDER; Type 0: Not a Combination Product 11/02/2015 09/29/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 11/02/2015 09/29/2021 Labeler - The Procter & Gamble Manufacturing Company (004238200)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.