CALM-ITO by Nartex Laboratorios Homeopaticos, S.A. de C.V.
CALM-ITO by
Drug Labeling and Warnings
CALM-ITO by is a Homeopathic medication manufactured, distributed, or labeled by Nartex Laboratorios Homeopaticos, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CALM-ITO- coffea cruda, cypripendium, hyoscyamus and matricaria chamomilla tablet
Nartex Laboratorios Homeopaticos, S.A. de C.V.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Chamomilla.................3X HPUS
Coffea Cruda...............3X HPUS
Cypripedium................3X HPUS
Hyoscayamus...............3X HPUS
Chamomilla.................3X HPUS*.............Oversensitivity and sleeplessness
Coffea Cruda...............3X HPUS*........................Anxiety and sleeplessness
Cypripedium................3X HPUS*................................Nervousness in kids
Hyoscayamus...............3X HPUS*.....................Insomnia, scaared wake-up
*The letters "HPUS" indicate that hte components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. X is an homeopathic dilution. See www.nartexlabsusa.com for more information. Active ingredients are prepared in accordance with the Homeopathic Pharmacopoeia of the United States, and are therefore non-toxic and have no known side effects.
Helps relieve symptoms such as:
- irritability
- crying
- oversensitivity
- restlessness
- sleeplessness
as per Materia Medica
Product uses are based on Homeopathic Materia Medica. These "Uses" have not been evaluated by the Food and Drug Administration. This product has not been clinically tested by Nartex Labs USA, Inc.
Do not use
- if you have an allergy or hypersensitivity to components of the formulathis product except under advise and supervision of a physician
- if you are allergic to milk or milk products
Distributed by:
Nartex Labs USA, Inc.
11711 Memorial Dr. #685
Houston TX 77024
www.nartexlabsusa.com
Product claims are based on Homeopathic Materia Medica. This product has not been clinically tested by Nartex.
Keep carton. It contains important information.
For your protection, this bottle has a seal around the neck. Attention: do not use if the neck seal is broken.
This product is HOMEOPATHIC. Its claims and efficacy are based on homeopathic research and clinical experience. This product has not been evaluated for efficacy by FDA with the same rules and procedures as those for other non-homeopathic drugs. For more information on homeopathy vists www.nartexlabsusa.com before using this product.
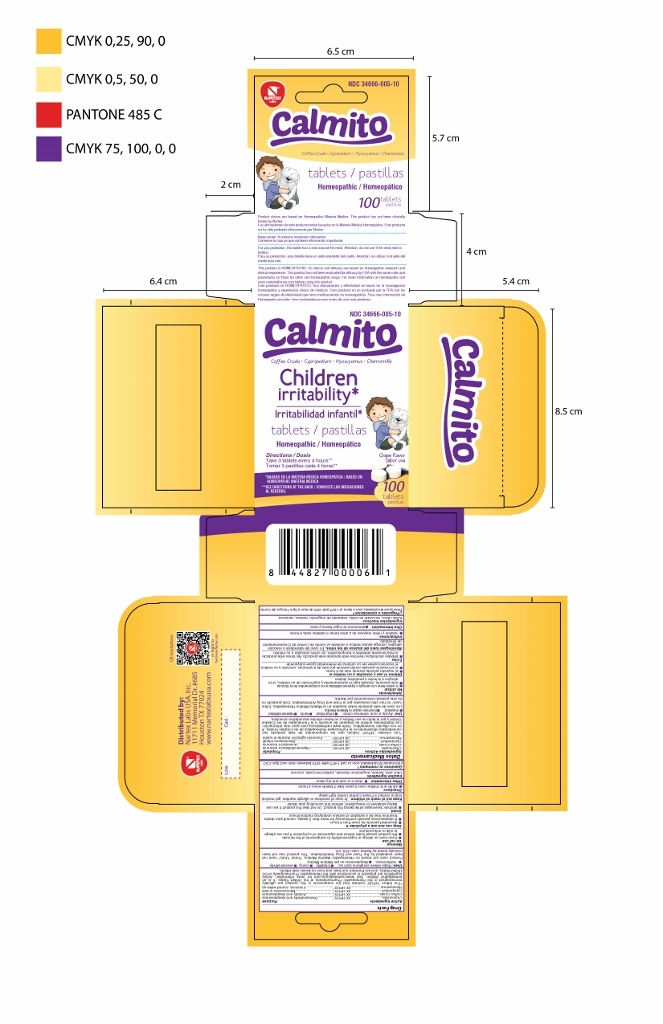
NDC: 34666-005-10
Calmito
Coffea cruda Cypripedium Hyoscyamus Chamomilla
Children irritability*
tablets
Homeopathic
Directions
Take 3 tablets every 4 hours**
Grape flavor
100 tablets
*BASED ON HOMEOPATHIC MATERIA MEDICA
**SEE DIRECTIONS AT THE BACK
| CALM-ITO
coffea cruda, cypripendium, hyoscyamus and matricaria chamomilla tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Nartex Laboratorios Homeopaticos, S.A. de C.V. (589914576) |
| Registrant - Nartex Laboratorios Homeopaticos, S.A. de C.V. (589914576) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nartex Laboratorios Homeopaticos, S.A. de C.V. | 589914576 | manufacture(34666-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.