METHOTREXATE injection, solution
METHOTREXATE by
Drug Labeling and Warnings
METHOTREXATE by is a Prescription medication manufactured, distributed, or labeled by Accord Healthcare Inc., Intas Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Methotrexate Injection safely and effectively. See full prescribing information for Methotrexate Injection.
METHOTREXATE injection, for intravenous use
Initial U.S. Approval: 1953WARNING: HYPERSENSITIVITY REACTIONS and SERIOUS ADVERSE REACTIONS
See full prescribing information for complete boxed warning.
- Methotrexate Injection is contraindicated in patients with a history of severe hypersensitivity reactions to methotrexate, including anaphylaxis ( 4, 5.1).
- Serious adverse reactions, including death, have been reported with methotrexate. Closely monitor for adverse reactions of the nervous system, kidneys, liver, bone marrow, gastrointestinal tract, lungs, and skin. Withhold or discontinue Methotrexate Injection as appropriate ( 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8).
INDICATIONS AND USAGE
Methotrexate Injection is a dihydrofolate reductase inhibitor indicated for the treatment of:
- adults and pediatric patients with acute lymphoblastic leukemia (ALL) as part of a combination chemotherapy regimen ( 1.1).
- adults with relapsed or refractory non-Hodgkin lymphoma as part of a combination chemotherapy regimen ( 1.2).
- adults and pediatric patients with Burkitt lymphoma as part of a combination chemotherapy regimen ( 1.3).
- adults and pediatric patients with osteosarcoma as part of a combination chemotherapy regimen ( 1.4).
- Limitations of Use: Methotrexate Injection is not indicated for non-oncology diseases ( 1.5).
DOSAGE AND ADMINISTRATION
- For intravenous use only. Methotrexate Injection is available as a solution at a concentration of 100 mg/mL. Verify the concentration prior to preparation and administration to avoid overdosage ( 2.1).
- See full prescribing information about monitoring and concomitant therapies for intermediate- and high-dose regimens ( 2.2).
- Acute Lymphoblastic Leukemia: The recommended dosage of Methotrexate Injection varies from 30 mg/m 2 to 5000 mg/m 2 intravenously as part of a combination chemotherapy regimen ( 2.3).
- Non-Hodgkin Lymphoma and Burkitt Lymphoma: The recommended dosage of Methotrexate Injection varies from 1000 mg/m 2 to 3000 mg/m 2 intravenously as single agent therapy or as part of a combination chemotherapy regimen ( 2.4).
- Osteosarcoma: The recommended dosage of Methotrexate Injection is typically 12 grams/m 2 (maximum: 20 grams per dose) as an intravenous infusion over 4 hours followed by leucovorin rescue in accordance with high-dose methotrexate regimen guidelines. Adjust subsequent doses based on observed peak serum methotrexate concentrations as appropriate ( 2.5).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- History of severe hypersensitivity to methotrexate ( 4)
WARNINGS AND PRECAUTIONS
- Serious Infections: Monitor patients for infection during and after treatment with Methotrexate Injection. Withhold or discontinue Methotrexate Injection for serious infections as appropriate ( 5.9).
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception ( 5.10, 8.1, 8.3).
- Secondary Malignancies: Can occur with methotrexate ( 5.11).
- Tumor Lysis Syndrome: Institute appropriate prophylactic measures in patients at risk for tumor lysis syndrome prior to initiation of Methotrexate Injection ( 5.12).
- Immunizations and Risks Associated with Live Vaccines: Administer immunizations according to best practice guidelines prior to and during treatment with Methotrexate Injection. Immunizations with live vaccines is not recommended ( 5.13).
ADVERSE REACTIONS
Common adverse reactions are: ulcerative stomatitis, leukopenia, nausea and abdominal distress ( 6).
To report SUSPECTED ADVERSE REACTIONS, contact Accord Healthcare Inc. at 1-866-941-7875 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Refer to the full prescribing information for information about drug interactions with methotrexate (7).
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed (8.2).
- Renal Impairment: Consider reducing the dose or discontinuing Methotrexate Injection for renal impairment as appropriate (8.6).
- Hepatic Impairment: Consider reducing the dose or discontinuing Methotrexate Injection for hepatic impairment as appropriate (8.7).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNINGS: HYPERSENSITIVITY REACTIONS and SERIOUS ADVERSE REACTIONS
1 INDICATIONS AND USAGE
1.1 Acute Lymphoblastic Leukemia
1.2 Non-Hodgkin Lymphoma
1.3 Burkitt Lymphoma
1.4 Osteosarcoma
1.5 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Recommended Monitoring and Concomitant Therapies to Promote Methotrexate Elimination and Decrease Toxicity for Intermediate- and High-Dose Regimens
2.3 Recommended Dosage for Acute Lymphoblastic Leukemia
2.4 Recommended Dosage for Non-Hodgkin Lymphoma
2.5 Recommended Dosage for Burkitt Lymphoma
2.6 Recommended Dosage for Osteosarcoma
2.7 Dosage Modifications for Adverse Reactions
2.8 Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Neurotoxicity
5.3 Renal Toxicity
5.4 Hepatotoxicity
5.5 Myelosuppression
5.6 Gastrointestinal Toxicity
5.7 Pulmonary Toxicity
5.8 Dermatologic Toxicity
5.9 Serious Infections
5.10 Embryo-Fetal Toxicity
5.11 Secondary Malignancies
5.12 Tumor Lysis Syndrome
5.13 Immunization and Risks Associated with Live Vaccines
5.14 Increased Risk of Adverse Reactions Due to Third-Space Accumulation
5.15 Increased Risk of Soft Tissue and Bone Toxicity with Concomitant Radiotherapy
5.16 Risk of Decreased Clinical Effectiveness with Folic Acid Supplementation
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Methotrexate
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNINGS: HYPERSENSITIVITY REACTIONS and SERIOUS ADVERSE REACTIONS
- Methotrexate Injection is contraindicated in patients with a history of severe hypersensitivity reactions to methotrexate, including anaphylaxis [ Contraindications (4), Warnings and Precautions (5.1)] .
- Serious adverse reactions, including death, have been reported with methotrexate. Closely monitor for adverse reactions of the nervous system, kidneys, liver, bone marrow, gastrointestinal tract, lungs, and skin. Withhold or discontinue Methotrexate Injection as appropriate [ Warnings and Precautions (5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8)] .
-
1 INDICATIONS AND USAGE
1.1 Acute Lymphoblastic Leukemia
Methotrexate Injection is indicated for the treatment of adults and pediatric patients with acute lymphoblastic leukemia (ALL) as part of a combination chemotherapy regimen.
1.2 Non-Hodgkin Lymphoma
Methotrexate Injection is indicated for the treatment of adults with relapsed or refractory non-Hodgkin lymphoma as part of a combination chemotherapy regimen.
1.3 Burkitt Lymphoma
Methotrexate Injection is indicated for the treatment of adults and pediatric patients with Burkitt lymphoma as part of a combination chemotherapy regimen.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
For intravenous use only. Methotrexate Injection is available as a preservative-free solution at a concentration of 100 mg/mL. Verify the concentration prior to preparation and administration to avoid overdosage.
2.2 Recommended Monitoring and Concomitant Therapies to Promote Methotrexate Elimination and Decrease Toxicity for Intermediate- and High-Dose Regimens
- Consider leucovorin rescue for patients who will receive Methotrexate Injection doses between 100 mg/m 2 to less than 500 mg/m 2 (e.g., intermediate-dose). Administer leucovorin rescue in patients who will receive Methotrexate Injection doses of 500 mg/m 2 or greater (e.g., high-dose). Refer to the leucovorin prescribing information for additional information.
- Consider the following for patients who will receive Methotrexate Injection doses between 100 mg/m
2 to less than 500 mg/m
2. Complete the following for patients who will receive Methotrexate Injection doses of 500 mg/m
2 or greater
[see
Warnings and Precautions (5.3)]
- Monitor serum creatinine and electrolytes at baseline and at least daily throughout treatment.
- Administer intravenous fluids starting before the first dose and continuing throughout treatment to maintain adequate hydration and urine output.
- Alkalinize urine starting before the first dose and continuing throughout treatment to maintain a urine pH of greater than 7.
- Monitor serum methotrexate concentrations and adjust hydration and leucovorin dosage as appropriate.
- Administer glucarpidase in patients who have toxic plasma methotrexate concentrations (> 1 micromole per liter) and delayed methotrexate clearance due to impaired renal function (refer to the glucarpidase prescribing information for additional information)
2.3 Recommended Dosage for Acute Lymphoblastic Leukemia
The recommended dosage of Methotrexate Injection varies from 30 mg/m 2 to 5,000 mg/m 2 intravenously as part of a combination chemotherapy regimen. Individualize the dose and schedule of Methotrexate Injection based on disease site, patient risk category, concurrent drugs, treatment phase and treatment response.
2.4 Recommended Dosage for Non-Hodgkin Lymphoma
The recommended dosage of Methotrexate Injection varies from 1,000 mg/m 2 to 3,000 mg/m 2 intravenously as part of a combination chemotherapy regimen. Individualize the dose and schedule of Methotrexate Injection based on disease state, patient risk category, concurrent drugs, treatment phase and treatment response.
2.5 Recommended Dosage for Burkitt Lymphoma
The recommended dosage of Methotrexate Injection varies from 1,000 mg/m 2 to 3,000 mg/m 2 intravenously as part of a combination chemotherapy regimen. Individualize the dose and schedule of Methotrexate Injection based on disease state, patient risk category, concurrent drugs, treatment phase and treatment response.
2.6 Recommended Dosage for Osteosarcoma
The recommended dosage of Methotrexate Injection is typically 12 grams/m 2 (maximum: 20 grams per dose) as an intravenous infusion over 4 hours followed by leucovorin rescue in accordance with high-dose methotrexate regimen guidelines [see Dosage and Administration (2.2)] . Adjust subsequent doses based on observed peak serum methotrexate concentrations as appropriate.
2.7 Dosage Modifications for Adverse Reactions
Discontinue Methotrexate Injection for
- Anaphylaxis or other severe hypersensitivity reactions [see Warnings and Precautions (5.1)]
- Lymphoproliferative disease [see Warnings and Precautions (5.11)]
Withhold, dose reduce or discontinue Methotrexate Injection as appropriate for:
- Myelosuppression [see Warnings and Precautions (5.5)]
Withhold or discontinue Methotrexate Injection as appropriate for:
- Neurotoxicity [see Warnings and Precautions (5.2)]
- Severe renal toxicity [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Severe gastrointestinal toxicity [see Warnings and Precautions (5.6)]
- Pulmonary toxicity [see Warnings and Precautions (5.7)]
- Severe dermatologic reactions [see Warnings and Precautions (5.8)]
- Serious infections [see Warnings and Precautions (5.9)]
2.8 Preparation
- Methotrexate Injection is available as a solution at a concentration of a 100 mg/mL. Verify the concentration prior to preparation and administration to avoid overdosage.
- Methotrexate Injection is a hazardous drug. Follow applicable special handling and disposable procedures. 1
- Methotrexate Injection may be further diluted immediately before use with an appropriate sterile, preservative-free medium such as 5% Dextrose Solution, USP or Sodium Chloride Injection, USP.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Methotrexate Injection is contraindicated in patients with a history of severe hypersensitivity to methotrexate. Reactions have included anaphylaxis [see Warnings and Precautions (5.1)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, can occur with methotrexate. Methotrexate Injection is contraindicated in patients with a history of severe hypersensitivity to methotrexate [see Contraindications (4), Adverse Reactions (6)] .
If anaphylaxis or another severe hypersensitivity reaction occurs, discontinue Methotrexate Injection [see Dosage and Administration (2.7)] .
5.2 Neurotoxicity
Methotrexate can cause severe acute and chronic neurotoxicity, which can be progressive, irreversible, and fatal [see Adverse Reactions (6)] .
Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with ALL who were treated with methotrexate at intravenous doses of 1,000 mg/m 2[see Use in Specific Populations (8.4)] .
Leukoencephalopathy can occur with intermediate- and high-dose regimens. The risk of leukoencephalopathy is increased with prior cranial radiation.
A transient acute stroke-like syndrome can occur with high-dose methotrexate. Clinical manifestations include confusion, hemiparesis, transient blindness, seizures, and coma.
Monitor patients for neurotoxicity. Withhold or discontinue Methotrexate Injection as appropriate [see Dosage and Administration (2.7)] .
5.3 Renal Toxicity
Methotrexate can cause renal toxicity, including irreversible acute renal failure [see Adverse Reactions (6)] .
Monitor renal function at baseline, periodically during treatment, and as clinically indicated. Withhold or discontinue Methotrexate Injection for severe renal toxicity as appropriate [see Dosage and Administration (2.7)] . Follow recommendations to promote methotrexate elimination and decrease risk of acute kidney injury and other methotrexate toxicities in patients who are receiving intermediate- or high-dose regimens [see Dosage and Administration (2.1)] .
5.4 Hepatotoxicity
Methotrexate can cause severe and potentially irreversible hepatotoxicity, including fibrosis, cirrhosis, and fatal liver failure [see Adverse Reactions (6)] . The safety of Methotrexate Injection in patients with hepatic disease is unknown.
The risk of hepatotoxicity is increased with heavy alcohol consumption.
Monitor liver tests at baseline, periodically during treatment, and as clinically indicated. Withhold or discontinue Methotrexate Injection as appropriate [see Dosage and Administration (2.7)] .
5.5 Myelosuppression
Methotrexate suppresses hematopoiesis and can cause severe and life-threatening pancytopenia, anemia, leukopenia, neutropenia, and thrombocytopenia [see Adverse Reactions (6)] .
Obtain complete blood counts at baseline, periodically during treatment, and as clinically indicated. Monitor patients for clinical complications of myelosuppression. Withhold, dose reduce, or discontinue Methotrexate Injection as appropriate [see Dosage and Administration (2.7)] .
5.6 Gastrointestinal Toxicity
Methotrexate can cause diarrhea, vomiting, stomatitis, hemorrhagic enteritis and fatal intestinal perforation [see Adverse Reactions (6)] . Patients with peptic ulcer disease or ulcerative colitis are at a greater risk of developing severe gastrointestinal adverse reactions [see Drug Interactions (7.1)] .
Withhold or discontinue Methotrexate Injection for severe gastrointestinal toxicity as appropriate [see Dosage and Administration (2.7)] .
5.7 Pulmonary Toxicity
Pulmonary toxicity, including acute or chronic interstitial pneumonitis and irreversible or fatal cases, can occur with methotrexate [see Adverse Reactions (6)] .
Monitor patients for signs of pulmonary toxicity. Withhold or discontinue Methotrexate Injection for pulmonary toxicity as appropriate [see Dosage and Administration (2.7)] .
5.8 Dermatologic Toxicity
Severe, including fatal dermatologic reactions, such as toxic epidermal necrolysis, Stevens- Johnson syndrome, exfoliative dermatitis, skin necrosis, and erythema multiforme, can occur with methotrexate [see Adverse Reactions (6)] .
Methotrexate can also cause radiation recall dermatitis and photodermatitis (sunburn) reactivation.
Monitor patients for signs of dermatologic toxicity. Withhold or discontinue Methotrexate Injection for severe dermatologic reactions as appropriate [see Dosage and Administration (2.7)] . Advise patients to avoid excessive sun exposure and use sun protection measures.
5.9 Serious Infections
Patients treated with methotrexate are at increased risk for developing life-threatening or fatal bacterial, fungal, or viral infections including opportunistic infections such as Pneumocystis jiroveci pneumonia, invasive fungal infections, hepatitis B reactivation, tuberculosis primary infection or reactivation, and disseminated Herpes zoster and cytomegalovirus infections [see Adverse Reactions (6)] .
Monitor patients for infection during and after treatment with Methotrexate Injection. Withhold or discontinue Methotrexate Injection for serious infections as appropriate [see Dosage and Administration (2.7)] .
5.10 Embryo-Fetal Toxicity
Based on published reports and its mechanism of action, Methotrexate Injection can cause fetal harm when administered to a pregnant woman.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Methotrexate Injection and for 6 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Methotrexate Injection and for 3 months after the final dose [see Use in Specific Populations (8.1, 8.3)] .
5.11 Secondary Malignancies
Secondary malignancies can occur with methotrexate [see Adverse Reactions (6)] .
In some cases, lymphoproliferative disease that occurred during therapy with methotrexate regressed completely following withdrawal of methotrexate. If lymphoproliferative disease occurs, discontinue Methotrexate Injection [see Dosage and Administration (2.7)] .
5.12 Tumor Lysis Syndrome
Methotrexate can induce tumor lysis syndrome in patients with rapidly growing tumors. Institute appropriate prophylactic measures in patients at risk for tumor lysis syndrome prior to initiation of Methotrexate Injection.
5.13 Immunization and Risks Associated with Live Vaccines
Disseminated infections following administration of live vaccines have been reported.
Administer immunizations according to current best practice guidelines prior to initiating Methotrexate Injection and then follow these guidelines for administering immunizations during treatment with Methotrexate Injection. Immunization with live vaccines is not recommended during treatment with Methotrexate Injection. The interval between live vaccines and initiation of Methotrexate Injection should be in accordance with the current best practice guidelines for patients on immunosuppressive therapies.
5.14 Increased Risk of Adverse Reactions Due to Third-Space Accumulation
Methotrexate accumulates in third-spaces (e.g., pleural effusions or ascites), which results in a prolonged elimination and increases the risk of adverse reactions. Evacuate significant third-space accumulations prior to Methotrexate Injection administration as appropriate.
5.15 Increased Risk of Soft Tissue and Bone Toxicity with Concomitant Radiotherapy
Concomitant radiotherapy increases the risk of soft tissue necrosis and osteonecrosis associated with methotrexate.
5.16 Risk of Decreased Clinical Effectiveness with Folic Acid Supplementation
Products containing folic acid or its derivatives may decrease the clinical effectiveness of methotrexate. Instruct patients not to take products containing folic acid or folinic acid unless directed to do so by their healthcare provider.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Neurotoxicity [see Warnings and Precautions (5.2)]
- Renal Toxicity [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Myelosuppression [see Warnings and Precautions (5.5)]
- Gastrointestinal Toxicity [see Warnings and Precautions (5.6)]
- Pulmonary toxicity [see Warnings and Precautions (5.7)]
- Dermatologic Toxicity [see Warnings and Precautions (5.8)]
- Serious infections [see Warnings and Precautions (5.9)]
- Secondary Malignancies [see Warnings and Precautions (5.11)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.12)]
The following adverse reactions associated with the use of methotrexate were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Common adverse reactions were: ulcerative stomatitis, leukopenia, nausea, and abdominal distress. Other frequently reported adverse reactions were: infection, malaise, fatigue, chills, fever, and dizziness.
Blood and lymphatic system disorders: aplastic anemia, lymphadenopathy, hypogammaglobulinemia
Cardiac disorders: thromboembolic events (including arterial thrombosis, cerebral thrombosis, deep vein thrombosis, retinal vein thrombosis, thrombophlebitis, pulmonary embolus pericarditis, pericardial effusion, hypotension, and sudden death)
Endocrine disorders: diabetes
Eye disorders: optic neuropathy, blurred vision, ocular irritation, conjunctivitis, xerophthalmia
Gastrointestinal disorders: hemorrhagic enteritis, intestinal perforation, gingivitis, pancreatitis, pharyngitis, hematemesis, melena, gastrointestinal ulceration and bleeding
Hepatobiliary disorders: acute hepatitis, fibrosis, cirrhosis, decreased serum albumin
Immune system disorders: anaphylaxis, vasculitis
Metabolism and nutrition disorders: hyperglycemia
Nervous system disorders: headaches, drowsiness, blurred vision, speech impairment (including dysarthria and aphasia), transient cognitive dysfunction, mood alteration, unusual cranial sensations, paresis, encephalopathy, convulsions
Renal and urinary disorders: azotemia, hematuria, proteinuria, cystitis
Reproductive system and breast disorders: defective oogenesis or spermatogenesis, loss of libido, impotence, gynecomastia, menstrual dysfunction
Respiratory, thoracic and mediastinal disorders: pulmonary fibrosis, respiratory failure, chronic interstitial obstructive pulmonary disease, pleuritic pain and thickening, alveolitis
Skin and subcutaneous disorders: toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, skin necrosis, erythema multiforme, erythematous rashes, pruritus, alopecia, skin ulceration, accelerated nodulosis, urticaria, pigmentary changes, ecchymosis, telangiectasia, photosensitivity, acne, furunculosis
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Methotrexate
Drugs that Increase Methotrexate Exposure
Coadministration of methotrexate with the following products may increase methotrexate plasma concentrations, which may increase the risk of severe methotrexate adverse reactions. In some cases, the coadministration of methotrexate with these products may also subsequently reduce active metabolite formation, which may decrease the clinical effectiveness of methotrexate. Increased organ specific adverse reactions may also occur when methotrexate is coadministered with hepatotoxic or nephrotoxic products.
If coadministration cannot be avoided, closely monitor for methotrexate adverse reactions when coadministered with:
- Penicillin or sulfonamide antibiotics
- Highly protein-bound drugs (e.g., oral anticoagulants, phenytoin, salicylates, sulfonamides, sulfonylureas, and tetracyclines)
- Proton pump inhibitors
- Weak acids (e.g., salicylates)
- Nephrotoxic products
- Probenecid
- Antifolate drugs (e.g., dapsone, pemetrexed, pyrimethamine and sulfonamides)
- Aspirin and other nonsteroidal anti-inflammatory drugs
- Hepatotoxic products
Nitrous Oxide
Coadministration of methotrexate with nitrous oxide anesthesia potentiates the effect of methotrexate on folate-dependent metabolic pathways, which may increase the risk of severe methotrexate adverse reactions. Avoid nitrous oxide anesthesia in patients receiving Methotrexate Injection. Consider alternative therapies in patients who have recently received nitrous oxide anesthesia.
Folic Acid
Coadministration of methotrexate with folic acid or its derivatives may decrease the clinical effectiveness of methotrexate. Methotrexate competes with reduced folates for active transport across cell membranes. Instruct patients to take folic or folinic acid only as directed by their healthcare provider [see Warnings and Precautions (5.16)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on published reports and its mechanism of action [see Clinical Pharmacology (12.1)] , Methotrexate Injection can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman (see Data). There are no animal data that meet current standards for nonclinical developmental toxicity studies. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Published data from cases, literature reviews, and observational studies report that methotrexate exposure during pregnancy is associated with an increased risk of embryo-fetal toxicity and fetal death. Methotrexate exposure during the first trimester of pregnancy is associated with an increased incidence of spontaneous abortions and multiple adverse developmental outcomes, including skull anomalies, facial dysmorphism, central nervous system abnormalities, limb abnormalities, and sometimes cardiac anomalies and intellectual impairment. Methotrexate exposure during the second and third trimesters of pregnancy is associated with intrauterine growth restriction and functional abnormalities. Because methotrexate is widely distributed and persists in the body for a prolonged period, there is a potential risk to the fetus from preconception methotrexate exposure.
A prospective multicenter study evaluated pregnancy outcomes in women taking methotrexate less than or equal to 30 mg per week after conception. The rate of miscarriage in pregnant women exposed to methotrexate was 42% (95% confidence interval [95% CI] 29, 59), which was higher than in unexposed patients with autoimmune disease (22%; 95% CI: 17, 30) and unexposed patients with non-autoimmune disease (17%; 95% CI: 13, 23). Of the live births, the rate of major birth defects in pregnant women exposed to methotrexate after conception was higher than in unexposed patients with autoimmune disease (adjusted odds ratio (OR) 1.8 [95% CI: 0.6, 6]) and unexposed patients with non-autoimmune disease (adjusted OR 3.1 [95% CI: 1, 10]). Major birth defects associated with pregnancies exposed to methotrexate after conception were not always consistent with methotrexate-associated adverse developmental outcomes.
8.2 Lactation
Risk Summary
Limited published literature report the presence of methotrexate in human milk in low amounts, with the highest breast milk to plasma concentration ratio reported to be 0.08:1. There are no data on the effects of methotrexate or its metabolites on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with Methotrexate Injection and for 1 week after the final dose.
8.3 Females and Males of Reproductive Potential
Methotrexate can cause malformations and fetal death at doses less than or equal to the recommended clinical doses [see Use in Specific Populations (8.1)] .
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating Methotrexate Injection [see Use in Specific Populations (8.1)] .
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with Methotrexate Injection and for 6 months after the final dose.
Males
Methotrexate Injection can cause chromosomal damage to sperm cells. Advise males with female partners of reproductive potential to use effective contraception during treatment with Methotrexate Injection and for 3 months after the final dose.
Infertility
Females
Based on published reports of female infertility after treatment with methotrexate, advise females of reproductive potential that Methotrexate Injection can cause impairment of fertility and menstrual dysfunction during and after cessation of therapy. It is not known if the infertility may be reversed in all affected females.
Males
Based on published reports of male infertility after treatment with methotrexate, advise males of reproductive potential that Methotrexate Injection can cause oligospermia or infertility during and after cessation of therapy. It is not known if the infertility may be reversed in all affected males.
8.4 Pediatric Use
The safety and effectiveness of Methotrexate Injection have been established in pediatric patients for the treatment of ALL, Burkitt lymphoma and osteosarcoma [see Indications and Usage (1), Dosage and Administration (2)] . No new safety signals have been observed in pediatric patients [see Adverse Reactions (6)] .
Serious neurotoxicity, frequently manifested as generalized or focal seizures, has been reported with unexpectedly increased frequency among pediatric patients with ALL who received methotrexate at intravenous doses of 1,000 mg/m 2[see Warnings and Precautions (5.2)] .
The safety and effectiveness of Methotrexate Injection have not been established in pediatric patients for non-Hodgkin lymphoma.
8.5 Geriatric Use
Clinical studies of methotrexate did not include sufficient numbers of patients aged 65 and older to determine whether they respond differently from younger patients.
8.6 Renal Impairment
Methotrexate elimination is reduced in patients with renal impairment [see Clinical Pharmacology (12.3)] . Patients with renal impairment are at increased risk for methotrexate adverse reactions.
Follow recommendations to promote methotrexate elimination and decrease risk of acute kidney injury and other methotrexate toxicities in patients who are receiving intermediate- or high-dose regimens [see Dosage and Administration (2.2)] . Consider reducing the dose or discontinuing Methotrexate Injection in patients with renal impairment as appropriate.
8.7 Hepatic Impairment
The pharmacokinetics and safety of methotrexate in patients with hepatic impairment is unknown. Patients with hepatic impairment may be at increased risk for methotrexate adverse reactions based on the elimination characteristics of methotrexate [see Clinical Pharmacology (12.3)] . Consider reducing the dose or discontinuing Methotrexate Injection in patients with hepatic impairment as appropriate.
-
10 OVERDOSAGE
Manifestations
Overdosage, including fatal overdosage, has occurred with methotrexate.
Manifestations of overdosage include adverse reactions reported at recommended dosages, particularly hematologic and gastrointestinal reactions (e.g., leukopenia, thrombocytopenia, anemia, pancytopenia, myelosuppression, mucositis, stomatitis, oral ulceration, nausea, vomiting, gastrointestinal ulceration, or gastrointestinal bleeding). In some cases, no symptoms were reported. Sepsis or septic shock, renal failure, and aplastic anemia were also reported.
Management
Leucovorin and levoleucovorin are indicated to diminish the toxicity and counteract the effect of overdosages of methotrexate. Administer leucovorin or levoleucovorin as soon as possible after overdosage (refer to the leucovorin or levoleucovorin prescribing information). Monitor serum methotrexate concentration closely to guide leucovorin or levoleucovorin therapy. Monitor serum creatinine concentrations closely, because high serum methotrexate concentrations may cause renal damage leading to acute renal failure.
Glucarpidase is indicated for the treatment of toxic methotrexate concentrations in patients with delayed methotrexate clearance due to impaired renal function (refer to the glucarpidase prescribing information). If glucarpidase is used, do not administer leucovorin within two hours before or after a dose of glucarpidase because leucovorin is a substrate for glucarpidase.
Hydration and urinary alkalinization may be necessary to prevent the precipitation of methotrexate and its metabolites in the renal tubules.
Neither hemodialysis nor peritoneal dialysis has been shown to improve methotrexate elimination; however, effective clearance of methotrexate has been reported with acute, intermittent hemodialysis using a high flux dialyzer.
-
11 DESCRIPTION
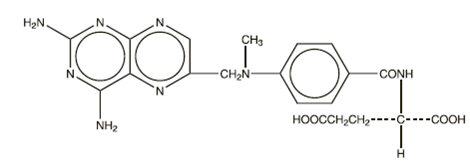
Methotrexate is a dihydrofolate reductase inhibitor with the chemical name of N-[4-[[(2,4-diamino-6-pteridinyl) methyl]methylamino]benzoyl]-L-glutamic acid. The molecular weight is 454.45 g/mol and the molecular formula is C 20H 22N 8O 5. The structural formula is:
Methotrexate is practically insoluble in water. Therefore, the pH of the methotrexate solution is adjusted to approximately 8.5 with sodium hydroxide in Water for Injection to produce Methotrexate Injection.
Methotrexate Injection for intravenous use is supplied as clear orange-yellow, sterile, preservative-free solution in clear glass single-dose vial sealed with gray rubber stopper and yellow aluminum flip-off seal. Each 100 mg/mL, 50 mL vial contains 5 grams methotrexate and the following inactive ingredient: sodium hydroxide to adjust the pH to approximately 8.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. Therefore, methotrexate interferes with DNA synthesis, repair, and cellular replication. Actively proliferating tissues such as malignant cells, bone marrow, fetal cells, buccal and intestinal mucosa, and cells of the urinary bladder are in general more sensitive to this effect of methotrexate.
12.3 Pharmacokinetics
Distribution
After intravenous administration, the initial volume of distribution is approximately 0.18 L/kg (18% of body weight) and steady-state volume of distribution is approximately 0.4 L/kg to 0.8 L/kg (40 to 80% of body weight).
Methotrexate competes with reduced folates for active transport across cell membranes by means of a single carrier-mediated active transport process. At serum concentrations greater than 100 micromolar, passive diffusion becomes a major pathway by which effective intracellular concentrations can be achieved.
Methotrexate in serum is approximately 50% protein bound.
Methotrexate does not penetrate the blood-cerebrospinal fluid barrier in therapeutic amounts when given intravenously.
Elimination
Following intravenous administration of high-dose methotrexate, the terminal half-life is 8 hours to 15 hours.
Metabolism
Methotrexate undergoes hepatic and intracellular metabolism to polyglutamated forms which can be converted back to methotrexate by hydrolase enzymes. These polyglutamates act as inhibitors of dihydrofolate reductase and thymidylate synthetase. Small amounts of methotrexate polyglutamates may remain in tissues for extended periods. The retention and prolonged drug action of these active metabolites vary among different cells, tissues and tumors. Methotrexate undergoes minor metabolism to 7-hydroxymethotrexate and accumulation may become significant at the high dosages. The aqueous solubility of 7-hydroxymethotrexate is 3- to 5-fold lower than the solubility of methotrexate.
Excretion
Renal excretion is the primary route of elimination and is dependent upon dosage and route of administration. With intravenous administration, 80% to 90% of the administered dose is excreted unchanged in the urine within 24 hours. There is limited biliary excretion amounting to 10% or less of the administered dose. Enterohepatic recirculation of methotrexate has been proposed.
Renal excretion occurs by glomerular filtration and active tubular secretion. Nonlinear elimination due to saturation of renal tubular reabsorption has been observed at doses between 7.5 mg and 30 mg.
Specific Populations
Pediatric Patients
In pediatric patients receiving high-dose methotrexate for ALL (5,000 mg/m 2 administered intravenously over 24 hours), serum methotrexate level decreased from a median level of 66 µmol/L during the first 12 hours post infusion, with a median half-life of 1.8 hours (range: 1.7 h to 2.3 h).
Patients with Renal impairment
The elimination half-life of methotrexate increases with the severity of renal impairment, with high inter-individual variability [see Use in Specific Populations (8.6)] .
- 13 NONCLINICAL TOXICOLOGY
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Methotrexate Injection is a clear orange-yellow, isotonic, sterile, preservative-free solution supplied in a carton containing 1 single-dose vial.
- 5 grams/50 mL (100 mg/mL) NDC: 16729-516-11
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
PROTECT FROM LIGHT.
Methotrexate Injection is a hazardous drug. Follow applicable special handling and disposable procedures. 1
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients of the potential risk of hypersensitivity and that Methotrexate Injection is contraindicated in patients with a history of severe hypersensitivity to methotrexate. Advise patients to seek immediate medical attention for signs or symptoms of a hypersensitivity reaction [see Warnings and Precautions (5.1)] .
Neurotoxicity
Advise patient to contact their healthcare provider for new or worsening neurological signs or symptoms [see Warnings and Precautions (5.2)] .
Renal Toxicity
Advise patients to immediately contact their healthcare provider for signs or symptoms of renal toxicity, such as marked increases or decreases in urinary output [see Warnings and Precautions (5.3)] .
Hepatotoxicity
Advise patients to report signs or symptoms of hepatotoxicity to their healthcare provider [see Warnings and Precautions (5.4)] .
Myelosuppression and Serious Infections
Advise patients to contact their healthcare provider for new onset fever, signs or symptoms of infection, easy bruising or persistent bleeding [see Warnings and Precautions (5.5, 5.9)] .
Gastrointestinal Toxicity
Advise patients to contact their healthcare provider if they develop new or worsening diarrhea, vomiting, or stomatitis. Advise patients to immediately contact their healthcare provider for high fever, rigors, persistent or severe abdominal pain, severe constipation, hematemesis, or melena [see Warnings and Precautions (5.6)] .
Pulmonary Toxicity
Advise patients to contact their healthcare provider for cough, fever, or dyspnea [see Warnings and Precautions (5.7)] .
Dermatologic Toxicity
Advise patients that Methotrexate Injection can cause serious skin rash and to immediately contact their healthcare provider for new or worsening skin rash. Advise patients to avoid excessive sun exposure and to use sun protection measures [see Warnings and Precautions (5.8)] .
Secondary Malignancies
Advise patients on the risk of secondary malignancies with Methotrexate Injection [see Warnings and Precautions (5.11)] .
Folic Acid Supplementation
Instruct patients not to take products containing folic acid or folinic acid unless directed to do so by their healthcare provider [see Warnings and Precautions (5.16)] .
Embryo-Fetal Toxicity
- Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.10), Use in Specific Populations (8.1)] .
- Advise females of reproductive potential to use effective contraception during treatment with Methotrexate Injection and for 6 months after the final dose [see Use in Specific Populations (8.3)] .
- Advise males of reproductive potential to use effective contraception during treatment with Methotrexate Injection and for 3 months after the final dose [see Use in Specific Populations (8.3)] .
Lactation
Advise women not to breastfeed during treatment with Methotrexate Injection and for 1 week after the final dose [see Use in Specific Populations (8.2)] .
Infertility
Advise females and males of reproductive potential that methotrexate may impair fertility [see Use in Specific Populations (8.3)] .
Drug Interactions
Advise patients to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7.1)] .
Manufactured For:
Accord Healthcare, Inc.
1009 Slater Road
Suite 210-B
Durham, NC 27703
USA.Manufactured By:
Intas Pharmaceuticals Limited
Plot No. 5 to 14, Pharmez
Nr. Village Matoda
Bavla Road, Ta.-Sanand
Dist. Ahmedabad-382 213
INDIA.
Issued August 2020
51 3693 0 720903
-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
NDC: 16729-516-11
50 mL Vial
Methotrexate Injection, USP
5g/50 mL
(100 mg/mL)
Cytotoxic Agent
Rx only
Single Dose Vial

-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
NDC: 16729-516-11
50 mL Vial
Methotrexate Injection, USP
Preservative Free
5g/50 mL
(100 mg/mL)
Cytotoxic Agent
Rx only
Single Dose Vial

-
INGREDIENTS AND APPEARANCE
METHOTREXATE
methotrexate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16729-516 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOTREXATE SODIUM (UNII: 3IG1E710ZN) (METHOTREXATE - UNII:YL5FZ2Y5U1) METHOTREXATE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16729-516-11 1 in 1 CARTON 08/01/2024 1 50 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214121 08/01/2024 Labeler - Accord Healthcare Inc. (604222237) Establishment Name Address ID/FEI Business Operations Intas Pharmaceuticals Limited 915837971 analysis(16729-516) , manufacture(16729-516)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.