POSACONAZOLE injection, solution
Posaconazole by
Drug Labeling and Warnings
Posaconazole by is a Prescription medication manufactured, distributed, or labeled by Fresensius Kabi USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POSACONAZOLE INJECTION safely and effectively. See full prescribing information for POSACONAZOLE INJECTION.
Posaconazole injection, for intravenous use
Initial U.S. Approval: 2006RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Posaconazole is an azole antifungal indicated as follows:
- Posaconazole injection is indicated for the treatment of invasive aspergillosis in adults and pediatric patients 13 years of age and older. (1.1)
-
Posaconazole is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy as follows: (1.2)
- Posaconazole injection: adults and pediatric patients 2 years of age and older
DOSAGE AND ADMINISTRATION
- Posaconazole injection must be administered through an in-line filter.
- Administer Posaconazole injection by intravenous infusion over approximately 90 minutes via a central venous line. (2.1)
- Do NOT administer Posaconazole injection as intravenous bolus injection. (2.1)
Table 1: Recommended Dosage in Adult Patients Indication Dosage Form, Dose, and Duration of Therapy Treatment of invasive Aspergillosis Posaconazole Injection:
Loading dose:
300 mg Posaconazole injection intravenously twice a day on the first day.
Maintenance dose:
300 mg Posaconazole injection intravenously once a day thereafter. Recommended total duration of therapy is 6 to 12 weeks. (2.2)Prophylaxis of Posaconazole Injection: invasive Loading dose: 300 mg Posaconazole injection Aspergillus and intravenously twice a day on the first day. Candida Maintenance dose: 300 mg Posaconazole injection intravenously once a day thereafter infections Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.2, 2.3) DOSAGE FORMS AND STRENGTHS
- Posaconazole injection: 300 mg per vial (18 mg per mL) in a single dose vial (3)
CONTRAINDICATIONS
- Known hypersensitivity to posaconazole or other azole antifungal agents. (4.1)
- Coadministration of Posaconazole with the following drugs is contraindicated; Posaconazole increases concentrations and toxicities of:
- Sirolimus: (4.2, 5.1, 7.1)
- CYP3A4 substrates (pimozide, quinidine): can result in QTc interval prolongation and cases of torsades de pointes (TdP) (4.3, 5.2, 7.2)
- HMG-CoA Reductase Inhibitors Primarily Metabolized through CYP3A4 (4.4, 7.3)
- Ergot alkaloids (4.5, 7.4)
- Venetoclax: in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) at initiation and during the ramp of phase (4.6, 5.10, 7.16)
WARNINGS AND PRECAUTIONS
- Calcineurin-Inhibitor Toxicity: Posaconazole increases concentrations of cyclosporine or tacrolimus; reduce dose of cyclosporine and tacrolimus and monitor concentrations frequently. (5.1)
- Arrhythmias and QTc Prolongation: Posaconazole has been shown to prolong the QTc interval and cause cases of TdP. Administer with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs known to prolong QTc interval and metabolized through CYP3A4. (5.2)
- Electrolyte Disturbances: Monitor and correct, especially those involving potassium (K+), magnesium (Mg++), and calcium (Ca++), before and during Posaconazole therapy. (5.3)
- Hepatic Toxicity: Elevations in liver tests may occur. Discontinuation should be considered in patients who develop abnormal liver tests or monitor liver tests during treatment. (5.4)
- Renal Impairment: Posaconazole injection should be avoided in patients with moderate or severe renal impairment (creatinine clearance <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Posaconazole injection. (5.5, 8.6)
- Concomitant Use with Midazolam: Posaconazole can prolong hypnotic/sedative effects. Monitor patients and benzodiazepine receptor antagonists should be available. (5.6, 7.5)
- Vincristine Toxicity: Concomitant administration of azole antifungals, including Posaconazole , with vincristine has been associated with neurotoxicity and other serious adverse reactions; reserve azole antifungals, including Posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options. (5.7, 7.10)
- Venetoclax Toxicity: Concomitant administration of Posaconazole with venetoclax may increase venetoclax toxicities, including the risk of tumor lysis syndrome, neutropenia, and serious infections; monitor for toxicity and reduce venetoclax dose. (4.6, 5.10, 7.16)
ADVERSE REACTIONS
- Adult Patients: Common adverse reactions in studies with Posaconazole in adults are diarrhea, nausea, fever, vomiting, headache, coughing, and hypokalemia. (6.1)
- Pediatric Patients: Common adverse reactions (incidence >20% receiving 6 mg/kg Posaconazole injection in a study in pediatric patients are pyrexia, febrile neutropenia, vomiting, mucosal inflammation, pruritus, hypertension, hypokalemia, and stomatitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
Interaction Drug Interaction Rifabutin, phenytoin, efavirenz Avoid coadministration unless the benefit outweighs the risks (7.6, 7.7, 7.8) Other drugs metabolized by CYP3A4
Consider dosage adjustment and monitor for adverse effects and toxicity (7.1, 7.10, 7.11) Digoxin Monitor digoxin plasma concentrations (7.12) Fosamprenavir Monitor for breakthrough fungal infections (7.6) USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Invasive Aspergillosis
1.2 Prophylaxis of Invasive Aspergillus and Candida Infections
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing Regimen in Adult Patients
2.3 Dosing Regimen in Pediatric Patients (ages 2 to less than 18 years of age)
2.4 Preparation, Intravenous Line Compatibility, and Administration of Posaconazole Injection
2.9 Dosage Adjustments in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Use with Sirolimus
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
4.5 Use with Ergot Alkaloids
4.6 Use with Venetoclax
5 WARNINGS AND PRECAUTIONS
5.1 Calcineurin-Inhibitor Toxicity
5.2 Arrhythmias and QT Prolongation
5.3 Electrolyte Disturbances
5.4 Hepatic Toxicity
5.5 Renal Impairment
5.6 Midazolam Toxicity
5.7 Vincristine Toxicity
5.10 Venetoclax Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Immunosuppressants Metabolized by CYP3A4
7.2 CYP3A4 Substrates
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
7.4 Ergot Alkaloids
7.5 Benzodiazepines Metabolized by CYP3A4
7.6 Anti-HIV Drugs
7.7 Rifabutin
7.8 Phenytoin
7.10 Vinca Alkaloids
7.11 Calcium Channel Blockers Metabolized by CYP3A4
7.12 Digoxin
7.14 Glipizide
7.16 Venetoclax
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Gender
8.9 Race
8.10 Weight
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Treatment of Invasive Aspergillosis with Posaconazole Injection and Noxafil® Delayed-Release Tablets
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Treatment of Invasive Aspergillosis
Posaconazole injection is indicated for the treatment of invasive aspergillosis in adults and pediatric patients 13 years of age and older.
1.2 Prophylaxis of Invasive Aspergillus and Candida Infections
Posaconazole is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus- host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy [see Clinical Studies (14.1)] as follows:
- Posaconazole injection: adults and pediatric patients 2 years of age and older
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Posaconazole Injection
- Administer via a central venous line, including a central venous catheter or peripherally inserted central catheter (PICC), by slow intravenous infusion over approximately 90 minutes [see Dosage and Administration (2.4)].
- If a central venous catheter is not available, Posaconazole injection may be administered through a peripheral venous catheter by slow intravenous infusion over 30 minutes only as a single dose in advance of central venous line placement or to bridge the period during which a central venous line is replaced or is in use for other intravenous treatment.
- When multiple dosing is required, the infusion should be done via a central venous line.
- Do NOT administer Posaconazole injection as an intravenous bolus injection.
2.2 Dosing Regimen in Adult Patients
Table 1: Dosing Regimens in Adult Patients
Indication
Dose and Frequency
Duration of TherapyTreatment of invasive Aspergillosis Posaconazole Injection:
Loading dose:
300 mg Posaconazole injection intravenously twice a day on the first day.
Maintenance dose:
300 mg Posaconazole injection intravenously once a day, starting on the second day.
Switching between the intravenous and delayed-release tablets is acceptable. A loading dose is not required when switching between formulations.Loading dose:
1 day
Maintenance dose:
Recommended total duration of therapy is 6 to 12 weeks.
Prophylaxis of invasive Aspergillus
and Candida infectionsPosaconazole Injection:
Loading dose:
300 mg Posaconazole injection intravenously twice a day on the first day.
Maintenance dose:
300 mg Posaconazole injection intravenously once a day thereafter.Loading dose:
1 day
Maintenance dose:
Duration of therapy is based on recovery from neutropenia or immunosuppression.2.3 Dosing Regimen in Pediatric Patients (ages 2 to less than 18 years of age)
The recommended dosing regimen of Posaconazole for pediatric patients 2 to less than 18 years of age is shown in Table 2 [see Clinical Pharmacology (12.3)].
Table 2: Posaconazole Injection Dosing Regimens for Pediatric Patients (ages 2 to less than 18 years of age) Recommended Pediatric Dosage and Formulation
Indication
Age
Injection
Duration of therapyProphylaxis of invasive Aspergillus
and Candida infectionsLess than or equal to 40 kg (2 to less than 18 years of age)
Greater than 40 kg (2 to less than 18 years of age)Loading dose:
6 mg/kg up to a maximum of 300 mg twice daily on the first day
Maintenance dose:
6 mg/kg up to a maximum of 300 mg once daily
Duration of therapy is based on recovery from neutropenia or immunosuppression.
Treatment of invasive Aspergillosis13 to less than 18 years of age regardless of weight. Loading dose:
300 mg Posaconazole injection intravenously twice a day on the first day.
Maintenance dose:
300 mg Posaconazole injection intravenously once a day, starting on the second day.
Switching between the intravenous and delayed-release tablets is acceptable. A loading dose is not required when switching between formulations.Loading dose:
1 day
Maintenance dose:
Recommended total duration of therapy is 6 to 12 weeks.2.4 Preparation, Intravenous Line Compatibility, and Administration of Posaconazole Injection
Preparation:
- Equilibrate the refrigerated vial of Posaconazole injection to room temperature.
- To prepare the required dose, aseptically transfer one vial (16.7 mL) of Posaconazole injection (containing 300 mg of posaconazole in solution) to an intravenous bag (or bottle) of a compatible admixture diluent (as described in Table 5), to achieve a final concentration of posaconazole that is between 1 mg/mL and 2 mg/mL. Use of other diluents is not recommended because they may result in particulate formation.
- Posaconazole injection is a single-dose sterile solution without preservatives. Discard any unused portion from the vial.
- Once admixed, the diluted solution of Posaconazole in the intravenous bag (or bottle) should be used immediately. If not used immediately, the solution can be stored up to 24 hours refrigerated 2 to 8°C (36 to 46°F). Discard any unused portion.
- Parenteral drug products should be inspected visually for particulate matter prior to administration, whenever solution and container permit. Once admixed, the solution of Posaconazole ranges from colorless to yellow. Variations of color within this range do not affect the quality of the product.
Intravenous Line Compatibility:
A study was conducted to evaluate physical compatibility of Posaconazole injection with injectable drug products and commonly used intravenous diluents during simulated Y-site infusion. Compatibility was determined through visual observations, measurement of particulate matter and turbidity. Compatible diluents and drug products are listed in Tables 5 and 6 below. Any diluents or drug products not listed in the tables below should not be co-administered through the same intravenous line (or cannula).
- Posaconazole injection can be infused at the same time through the same intravenous line (or cannula) with the following compatible diluents:
Table 5: Compatible Diluents 0.45% sodium chloride 0.9% sodium chloride 5% dextrose in water 5% dextrose and 0.45% sodium chloride 5% dextrose and 0.9% sodium chloride 5% dextrose and 20 mEq potassium chloride - Posaconazole injection can be infused at the same time through the same intravenous line (or cannula) with the following drug products prepared in 5% dextrose in water or sodium chloride 0.9%. Co-administration of drug products prepared in other diluents may result in particulate formation.
Table 6: Compatible Drugs Amikacin sulfate Caspofungin Ciprofloxacin Daptomycin Dobutamine hydrochloride Famotidine Filgrastim Gentamicin sulfate Hydromorphone hydrochloride Levofloxacin Lorazepam Meropenem Micafungin Morphine sulfate Norepinephrine bitartrate Potassium chloride Vancomycin hydrochloride Incompatible Diluents:
Posaconazole injection must not be diluted with the following diluents:
Lactated Ringer's solution
5% dextrose with Lactated Ringer's solution
4.2% sodium bicarbonate
Administration:
- Posaconazole injection must be administered through a 0.22-micron polyethersulfone (PES) or polyvinylidene difluoride (PVDF) filter.
- Administer via a central venous line, including a central venous catheter or PICC by slow infusion over approximately 90 minutes. Posaconazole injection is not for bolus administration.
- If a central venous catheter is not available, Posaconazole injection may be administered through a peripheral venous catheter only as a single dose in advance of central venous line placement or to bridge the period during which a central venous line is replaced or is in use for other treatment.
- When multiple dosing is required, the infusion should be done via a central venous line. When administered through a peripheral venous catheter, the infusion should be administered over approximately 30 minutes. Note: In clinical trials, multiple peripheral infusions given through the same vein resulted in infusion site reactions [see Adverse Reactions (6.1)].
2.9 Dosage Adjustments in Patients with Renal Impairment
- Posaconazole injection should be avoided in patients with moderate or severe renal impairment (eGFR <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Posaconazole injection.
- In patients with moderate or severe renal impairment (estimated glomerular filtration rate (eGFR) <50 mL/min), receiving the Posaconazole injection, accumulation of the intravenous vehicle, Betadex Sulfobutyl Ether Sodium (SBECD), is expected to occur. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral Posaconazole therapy.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Posaconazole is contraindicated in persons with known hypersensitivity to posaconazole or other azole antifungal agents.
4.2 Use with Sirolimus
Posaconazole is contraindicated with sirolimus. Concomitant administration of Posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
Posaconazole is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of Posaconazole with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
Coadministration with the HMG-CoA reductase inhibitors that are primarily metabolized through CYP3A4 (e.g., atorvastatin, lovastatin, and simvastatin) is contraindicated since increased plasma concentration of these drugs can lead to rhabdomyolysis [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
4.5 Use with Ergot Alkaloids
Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism [see Drug Interactions (7.4)].
4.6 Use with Venetoclax
Coadministration of Posaconzole with venetoclax at initiation and during the ramp-up phase is contraindicated in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) due to the potential for increased risk of tumor lysis syndrome [see Warnings and Precautions (5.10) and Drug Interactions (7.16)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Calcineurin-Inhibitor Toxicity
Concomitant administration of Posaconazole with cyclosporine or tacrolimus increases the whole blood trough concentrations of these calcineurin-inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Nephrotoxicity and leukoencephalopathy (including deaths) have been reported in clinical efficacy studies in patients with elevated cyclosporine or tacrolimus concentrations. Frequent monitoring of tacrolimus or cyclosporine whole blood trough concentrations should be performed during and at discontinuation of Posaconazole treatment and the tacrolimus or cyclosporine dose adjusted accordingly.
5.2 Arrhythmias and QT Prolongation
Some azoles, including Posaconazole, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, cases of torsades de pointes have been reported in patients taking Posaconazole.
Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18-85 years of age) administered Noxafil® oral suspension 400 mg twice daily with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc(F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc(F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered Posaconazole had a QTc(F) interval ≥500 msec or an increase ≥60 msec in their QTc(F) interval from baseline.
Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)].
5.3 Electrolyte Disturbances
Electrolyte disturbances, especially those involving potassium, magnesium or calcium levels, should be monitored and corrected as necessary before and during Posaconazole therapy.
5.4 Hepatic Toxicity
Hepatic reactions (e.g., mild to moderate elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and/or clinical hepatitis) have been reported in clinical trials. The elevations in liver tests were generally reversible on discontinuation of therapy, and in some instances these tests normalized without drug interruption. Cases of more severe hepatic reactions including cholestasis or hepatic failure including deaths have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with Posaconazole. These severe hepatic reactions were seen primarily in subjects receiving the Noxafil® oral suspension 800 mg daily (400 mg twice daily or 200 mg four times a day) in clinical trials.
Liver tests should be evaluated at the start of and during the course of Posaconazole therapy. Patients who develop abnormal liver tests during Posaconazole therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver tests and bilirubin). Discontinuation of Posaconazole must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to Posaconazole.
5.5 Renal Impairment
Posaconazole injection should be avoided in patients with moderate or severe renal impairment (eGFR <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Posaconazole injection. In patients with moderate or severe renal impairment (eGFR <50 mL/min), receiving the Posaconazole injection, accumulation of the intravenous vehicle, SBECD, is expected to occur. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral Posaconazole therapy [see Dosage and Administration (2.9) and Use in Specific Populations (8.6)].
5.6 Midazolam Toxicity
Concomitant administration of Posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Patients must be monitored closely for adverse effects associated with high plasma concentrations of midazolam and benzodiazepine receptor antagonists must be available to reverse these effects [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
5.7 Vincristine Toxicity
Concomitant administration of azole antifungals, including Posaconazole, with vincristine has been associated with neurotoxicity and other serious adverse reactions, including seizures, peripheral neuropathy, syndrome of inappropriate antidiuretic hormone secretion, and paralytic ileus. Reserve azole antifungals, including Posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options [see Drug Interactions (7.10)].
5.10 Venetoclax Toxicity
Concomitant administration of Posaconazole, a strong CYP3A4 inhibitor, with venetoclax may increase venetoclax toxicities, including the risk of tumor lysis syndrome (TLS), neutropenia, and serious infections. In patients with CLL/SLL, administration of Posaconazole during initiation and the ramp-up phase of venetoclax is contraindicated [see Contraindications (4.6)]. Refer to the venetoclax labeling for safety monitoring and dose reduction in the steady daily dosing phase in CLL/SLL patients.
For patients with acute myeloid leukemia (AML), dose reduction and safety monitoring are recommended across all dosing phases when coadministering Posaconazole with venetoclax [see Drug Interactions (7.16)]. Refer to the venetoclax prescribing information for dosing instructions.
-
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
- Hypersensitivity [see Contraindications (4.1)]
- Arrhythmias and QT Prolongation [see Warnings and Precautions (5.2)]
- Hepatic Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of Posaconazole cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Adults
Clinical Trial Experience with Posaconazole Injection and Noxafil® Delayed-Release Tablets for the Treatment of Invasive Aspergillosis
The safety of Posaconazole injection and Noxafil® delayed-release tablet was assessed in a randomized, double-blind, active-controlled clinical study of Posaconazole injection and Noxafil® delayed-release tablets versus voriconazole for treatment of invasive aspergillosis (Aspergillosis Treatment Study). A total of 575 (288 in Posaconazole arm, 287 in voriconazole arm) adult and pediatric patients 13 years of age and older with proven, probable or possible invasive aspergillosis were included. The median duration of treatment was 67 days for Posaconazole injection or Noxafil® delayed-release tablet and 64 days for voriconazole, with 55% to 60% of subjects starting treatment with the IV formulation of either drug. The median duration of the first instance of IV treatment (before switching to oral treatment or discontinuing or completing study treatment) was 9 days for both groups. Table 7 presents adverse reactions reported at an incidence of ≥10% in either one of the groups in Aspergillosis Treatment Study.
Adverse reactions leading to treatment discontinuation were reported for 33.9% of subjects. The most commonly reported adverse reactions (>2% of subjects) leading to treatment discontinuation were septic shock, respiratory failure, and bronchopulmonary aspergillosis in the Posaconazole arm, and septic shock and acute myeloid leukemia in the voriconazole arm.
Table 7: Posaconazole Invasive Aspergillosis Treatment Study: Adverse Reactions in at Least 10% of Subjects Treated with Posaconazole Injection or Noxafil® Delayed-Release Tablets System Organ Class Posaconazole injection or tablet (N = 288), n (%) Voriconazole injection or oral (N = 287), n (%) Blood and lymphatic system disorders Anemia 25 (8.7) 29 (10.1) Febrile neutropenia 42 (14.6) 38 (13.2) Gastrointestinal disorders Abdominal pain 29 (10.1) 24 (8.4) Constipation 32 (11.1) 23 (8.0) Diarrhea 52 (18.1) 52 (18.1) Nausea 65 (22.6) 51 (17.8) Vomiting 52 (18.1) 39 (13.6) General disorders and administration site conditions Edema peripheral 32 (11.1) 24 (8.4) Pyrexia 81 (28.1) 72 (25.1) Infections and infestations Pneumonia 36 (12.5) 26 (9.1) Investigations Alanine aminotransferase increased 42 (14.6) 37 (12.9) Aspartate aminotransferase increased 38 (13.2) 36 (12.5) Blood alkaline phosphatase increased 21 (7.3) 29 (10.1) Metabolism and nutrition disorders Hypokalemia 82 (28.5) 49 (17.1) Hypomagnesemia 29 (10.1) 18 (6.3) Nervous system disorders Headache 35 (12.2) 25 (8.7) Respiratory, thoracic and mediastinal disorders Cough 30 (10.4) 24 (8.4) Epistaxis 32 (11.1) 17 (5.9) The most frequently reported adverse reactions in the Posaconazole-treated group were pyrexia (28%), hypokalemia (28%), and nausea (23%).
Clinical Trial Experience with Posaconazole Injection for Prophylaxis
Multiple doses of Posaconazole injection administered via a peripheral venous catheter were associated with thrombophlebitis (60% incidence). Therefore, in subsequent studies, Posaconazole injection was administered via central venous catheter.
The safety of Posaconazole injection has been assessed in 268 patients in a clinical trial. Patients were enrolled in a non-comparative pharmacokinetic and safety trial of Posaconazole injection when given as antifungal prophylaxis (Posaconazole Injection Study). Patients were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, GVHD, and post HSCT. This patient population was 55% male, had a mean age of 51 years (range 18-82 years, 19% of patients were ≥65 years of age), and were 95% white and 8% Hispanic. Ten patients received a single dose of 200 mg Posaconazole injection, 21 patients received 200 mg daily dose for a median of 14 days, and 237 patients received 300 mg daily dose for a median of 9 days.
Table 8 presents adverse reactions observed in patients treated with Posaconazole injection 300 mg daily dose in the Posaconazole Injection Study. Each patient received a loading dose, 300 mg twice on Day 1. Following Posaconazole intravenous therapy, patients received Noxafil® oral suspension to complete 28 days of total Posaconazole therapy.
Table 8: Posaconazole Injection Study: Adverse Reactions in at Least 10% of Subjects Treated with Posaconazole Injection 300 mg Daily Dose *Adverse reactions reported in patients with an onset during the Posaconazole intravenous dosing phase of the study.
†Adverse reactions reported with an onset at any time during the study in patients who were treated for up to 28 days of Posaconazole therapy.
Body System
Posaconazole Injection Treatment Phase n=237 (%)*
Posaconazole Injection Treatment Phase or Subsequent Noxafil® Oral Suspension Treatment Phase n=237 (%)†
Subjects Reporting any Adverse Reaction
220
(93)
235
(99)
Blood and Lymphatic System Disorder
Anemia
16
(7)
23
(10)
Thrombocytopenia
17
(7)
25
(11)
Gastrointestinal Disorders
Abdominal Pain Upper
15
(6)
25
(11)
Abdominal Pain
30
(13)
41
(17)
Constipation
18
(8)
31
(13)
Diarrhea
75
(32)
93
(39)
Nausea
46
(19)
70
(30)
Vomiting
29
(12)
45
(19)
General Disorders and Administration Site Conditions
Fatigue
19
(8)
24
(10)
Chills
28
(12)
38
(16)
Edema Peripheral
28
(12)
35
(15)
Pyrexia
49
(21)
73
(31)
Metabolism and Nutrition Disorders
Decreased appetite
23
(10)
29
(12)
Hypokalemia
51
(22)
67
(28)
Hypomagnesemia
25
(11)
30
(13)
Nervous System Disorders
Headache
33
(14)
49
(21)
Respiratory, Thoracic and Mediastinal Disorders
Cough
21
(9)
31
(13)
Dyspnea
16
(7)
24
(10)
Epistaxis
34
(14)
40
(17)
Skin and Subcutaneous Tissue Disorders
Petechiae
20
(8)
24
(10)
Rash
35
(15)
56
(24)
Vascular Disorders
Hypertension
20
(8)
26
(11)The most frequently reported adverse reactions with an onset during the Posaconazole intravenous phase of dosing with 300 mg once daily were diarrhea (32%), hypokalemia (22%), pyrexia (21%), and nausea (19%). These adverse reactions were consistent with those seen in studies with Noxafil® oral suspension.
Clinical Trial Experience in Pediatrics
Clinical Trial Experience in Pediatric Patients (2 to less than 18 Years of Age)
The safety of Posaconazole injection and Noxafil® PowderMix for delayed-release oral suspension for prophylaxis of invasive fungal infections has been assessed in an open label uncontrolled dose-ranging PK and safety study (Posaconazole injection/ Noxafil® PowderMix for delayed-release oral suspension Pediatric Study 1, NCT02452034); hereinafter referred to as Posaconazole Pediatric Study) in 115 immunocompromised pediatric patients 2 to less than 18 years of age with known or expected neutropenia. Posaconazole injection and Noxafil® PowderMix for delayed-release oral suspension was administered at daily doses of up to 6 mg/kg (twice daily on day 1) in three dose cohorts. All 115 subjects initially received Posaconazole injection for at least 7 days, and 63 subjects were transitioned to Noxafil® PowderMix for delayed-release oral suspension. The mean overall treatment duration for all treated subjects was 20.6 days with 14.3 days (range: 1 to 28 days) on Posaconazole injection and 11.6 days (range: 2 to 18 days) on Noxafil® PowderMix for delayed-release oral suspension [see Clinical Pharmacology (12.3)].
Table 15 presents adverse reactions observed in greater than or equal to 10% of pediatric patients treated with Posaconazole in the Posaconazole Pediatric Study.
Reported adverse reaction profile of Posaconazole in pediatric patients was consistent with the safety profile of Posaconazole in adults. The most common adverse reactions (occurring in greater than 20% of pediatric patients receiving 6 mg/kg Posaconazole injection and Noxafil® PowderMix for delayed-release oral suspension daily dose) were pyrexia, febrile neutropenia, vomiting, mucosal inflammation, pruritus, hypertension, hypokalemia, and stomatitis.
Table 15: Adverse Reactions in at Least 10% of Pediatric Patients Treated with Posaconazole Injection and Noxafil® PowderMix for Delayed-Release Oral Suspension
Adverse Reaction
Posaconazole Injection and
Noxafil® PowderMix for Delayed-Release Oral Suspension
6 mg/kg Dose Cohort n=49 (%)
Posaconazole Injection and
Noxafil® PowderMix for Delayed-Release Oral Suspension
All Dose Cohorts n=115 (%)Pyrexia 16 (33) 50 (43) Febrile neutropenia 15 (31) 25 (22) Vomiting 12 (24) 30 (26) Mucosal inflammation 11 (22) 32 (28) Pruritus 11 (22) 18 (16) Hypertension 10 (20) 20 (17) Hypokalemia 10 (20) 16 (14) Stomatitis 10 (20) 13 (11) Diarrhea 9 (18) 25 (22) Nausea 9 (18) 18 (16) Abdominal pain 8 (16) 20 (17) Decreased appetite 7 (14) 17 (15) Rash 7 (14) 18 (16) Alanine aminotransferase increased 6 (12) 8 (7) Headache 6 (12) 16 (14) Aspartate aminotransferase increased 5 (10) 8 (7) The number of patients receiving Posaconazole in the Posaconazole Pediatric Study who had changes in liver tests from Grade 0, 1, or 2 at baseline to Grade 3 or 4 is presented in Table 16.
Table 16: Posaconazole Pediatric Study: Changes in Liver Tests from CTC Grade 0, 1, or 2 at Baseline to Grade 3 or 4 *Change from Grade 0 to 2 at baseline to Grade 3 or 4 during the study. These data are presented in the form X/Y, where X represents the number of patients who met the criterion as indicated, and Y represents the number of patients who had a baseline observation and at least one post-baseline observation.
CTC = Common Toxicity Criteria; AST= Aspartate Aminotransferase; ALT= Alanine Aminotransferase
Number (%) of Patients with Change*
Pediatric Study 1
Laboratory ParameterPosaconazole Injection and Noxafil® PowderMix for Delayed- Release Oral Suspension (6 mg/kg daily)
n=49 (%)AST 2/49 (4) ALT 3/49 (6) Bilirubin 0/48 (0) Alkaline Phosphatase 0/48 (0) 6.2 Postmarketing Experience
The following adverse reaction has been identified during the post-approval use of Posaconazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
Endocrine Disorders: Pseudoaldosteronism
-
7 DRUG INTERACTIONS
Posaconazole is primarily metabolized via UDP glucuronosyltransferase and is a substrate of p- glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Coadministration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole [see Clinical Pharmacology (12.3)].
The following information was derived from data with Noxafil® oral suspension or early tablet formulation unless otherwise noted. All drug interactions with Noxafil® oral suspension, except for those that affect the absorption of posaconazole (via gastric pH and motility), are considered relevant to Posaconazole injection, Noxafil® delayed-release tablet, and Noxafil® PowderMix for delayed-release oral suspension as well.
7.1 Immunosuppressants Metabolized by CYP3A4
Sirolimus: Concomitant administration of Posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity. Therefore, posaconazole is contraindicated with sirolimus [see Contraindications (4.2) and Clinical Pharmacology (12.3)].
Tacrolimus: Posaconazole has been shown to significantly increase the Cmax and AUC of tacrolimus. At initiation of posaconazole treatment, reduce the tacrolimus dose to approximately one-third of the original dose. Frequent monitoring of tacrolimus whole blood trough concentrations should be performed during and at discontinuation of Posaconazole treatment and the tacrolimus dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
Cyclosporine: Posaconazole has been shown to increase cyclosporine whole blood concentrations in heart transplant patients upon initiation of Posaconazole treatment. It is recommended to reduce cyclosporine dose to approximately three-fourths of the original dose upon initiation of Posaconazole treatment. Frequent monitoring of cyclosporine whole blood trough concentrations should be performed during and at discontinuation of Posaconazole treatment and the cyclosporine dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 CYP3A4 Substrates
Concomitant administration of Posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, Posaconazole is contraindicated with these drugs [see Contraindications (4.3) and Warnings and Precautions (5.2)].
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
Concomitant administration of Posaconazole with simvastatin increases the simvastatin plasma concentrations by approximately 10-fold. Therefore, Posaconazole is contraindicated with HMG-CoA reductase inhibitors primarily metabolized through CYP3A4 [see Contraindications (4.4) and Clinical Pharmacology (12.3)].
7.4 Ergot Alkaloids
Most of the ergot alkaloids are substrates of CYP3A4. Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism. Therefore, Posaconazole is contraindicated with ergot alkaloids [see Contraindications (4.5)].
7.5 Benzodiazepines Metabolized by CYP3A4
Concomitant administration of Posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant use of Posaconazole and other benzodiazepines metabolized by CYP3A4 (e.g., alprazolam, triazolam) could result in increased plasma concentrations of these benzodiazepines. Patients must be monitored closely for adverse effects associated with high plasma concentrations of benzodiazepines metabolized by CYP3A4 and benzodiazepine receptor antagonists must be available to reverse these effects [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.6 Anti-HIV Drugs
Efavirenz: Efavirenz induces UDP-glucuronidase and significantly decreases posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. It is recommended to avoid concomitant use of efavirenz with Posaconazole unless the benefit outweighs the risks.
Ritonavir and Atazanavir: Ritonavir and atazanavir are metabolized by CYP3A4 and Posaconazole increases plasma concentrations of these drugs [see Clinical Pharmacology (12.3)]. Frequent monitoring of adverse effects and toxicity of ritonavir and atazanavir should be performed during coadministration with Posaconazole.
Fosamprenavir: Combining fosamprenavir with Posaconazole may lead to decreased posaconazole plasma concentrations. If concomitant administration is required, close monitoring for breakthrough fungal infections is recommended [see Clinical Pharmacology (12.3)].
7.7 Rifabutin
Rifabutin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Rifabutin is also metabolized by CYP3A4. Therefore, coadministration of rifabutin with Posaconazole increases rifabutin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of Posaconazole and rifabutin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections as well as frequent monitoring of full blood counts and adverse reactions due to increased rifabutin plasma concentrations (e.g., uveitis, leukopenia) are recommended.
7.8 Phenytoin
Phenytoin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Phenytoin is also metabolized by CYP3A4. Therefore, coadministration of phenytoin with Posaconazole increases phenytoin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of Posaconazole and phenytoin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections is recommended and frequent monitoring of phenytoin concentrations should be performed while coadministered with Posaconazole and dose reduction of phenytoin should be considered.
7.10 Vinca Alkaloids
Most of the vinca alkaloids (e.g., vincristine and vinblastine) are substrates of CYP3A4. Concomitant administration of azole antifungals, including Posaconazole, with vincristine has been associated with serious adverse reactions [see Warnings and Precautions (5.7)]. Posaconazole may increase the plasma concentrations of vinca alkaloids which may lead to neurotoxicity and other serious adverse reactions. Therefore, reserve azole antifungals, including Posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options.
7.11 Calcium Channel Blockers Metabolized by CYP3A4
Posaconazole may increase the plasma concentrations of calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Frequent monitoring for adverse reactions and toxicity related to calcium channel blockers is recommended during coadministration. Dose reduction of calcium channel blockers may be needed.
7.12 Digoxin
Increased plasma concentrations of digoxin have been reported in patients receiving digoxin and Posaconazole. Therefore, monitoring of digoxin plasma concentrations is recommended during coadministration.
7.14 Glipizide
Although no dosage adjustment of glipizide is required, it is recommended to monitor glucose concentrations when Posaconazole and glipizide are concomitantly used.
7.16 Venetoclax
Concomitant use of venetoclax (a CYP3A4 substrate) with posaconazole increases venetoclax (cmax and AUC 0-INF, which may increase venetoclax toxicities [see Contraindications (4.6), Warnings and Precautions (5.10)]. Refer to the venetoclax prescribing information for more information on the dosing instructions and the extent of increase in venetoclax exposure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal data, Posaconazole may cause fetal harm when administered to pregnant women. Available data for use of Posaconazole in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, skeletal malformations (cranial malformations and missing ribs) and maternal toxicity (reduced food consumption and reduced body weight gain) were observed when posaconazole was dosed orally to pregnant rats during organogenesis at doses ≥1.4 times the 400 mg twice daily oral suspension regimen based on steady-state plasma concentrations of Posaconazole in healthy volunteers. In pregnant rabbits dosed orally during organogenesis, increased resorptions, reduced litter size, and reduced body weight gain of females were seen at doses 5 times the exposure achieved with the 400 mg twice daily oral suspension regimen. Doses of ≥ 3 times the clinical exposure caused an increase in resorptions in these rabbits (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Posaconazole resulted in maternal toxicity (reduced food consumption and reduced body weight gain) and skeletal malformations (cranial malformations and missing ribs) when given orally to pregnant rats during organogenesis (Gestational Days 6 through 15) at doses ≥27 mg/kg (≥1.4 times the 400 mg twice daily oral suspension regimen based on steady-state plasma concentrations of drug in healthy volunteers). The no-effect dose for malformations and maternal toxicity in rats was 9 mg/kg, which is 0.7 times the exposure achieved with the 400 mg twice daily oral suspension regimen. No malformations were seen in rabbits dosed during organogenesis (Gestational Days 7 through 19) at doses up to 80 mg/kg (5 times the exposure achieved with the 400 mg twice daily oral suspension regimen). In the rabbit, the no-effect dose was 20 mg/kg, while high doses of 40 mg/kg and 80 mg/kg (3 or 5 times the clinical exposure) caused an increase in resorptions. In rabbits dosed at 80 mg/kg, a reduction in body weight gain of females and a reduction in litter size were seen.
8.2 Lactation
Risk Summary
There are no data on the presence of posaconazole in human milk, the effects on the breastfed infant, or the effects on milk production. Posaconazole is excreted in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Posaconazole and any potential adverse effects on the breastfed child from Posaconazole or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Posaconazole injection for the prophylaxis of invasive Aspergillus and Candida infections have been established in pediatric patients aged 2 and older who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with GVHD or those with hematologic malignancies with prolonged neutropenia from chemotherapy.
The safety and effectiveness of Posaconazole injection for the treatment of invasive aspergillosis have been established in pediatric patients aged 13 years and older.
Use of Posaconazole in these age groups is supported by evidence from adequate and well-controlled studies of Posaconazole in adult and pediatric patients and additional pharmacokinetic and safety data in pediatric patients 2 years of age and older [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14)].
The safety and effectiveness of Posaconazole have not been established in pediatric patients younger than 2 years of age.
8.5 Geriatric Use
No overall differences in the safety of Posaconazole injection were observed between geriatric patients and younger adult patients in the clinical trials; therefore, no dosage adjustment is recommended for any formulation of Posaconazole in geriatric patients. No clinically meaningful differences in the pharmacokinetics of Posaconazole were observed in geriatric patients compared to younger adult patients during clinical trials [see Clinical Pharmacology (12.3)].
Of the 279 patients treated with Posaconazole injection in the Posaconazole Injection Study, 52 (19%) were greater than 65 years of age. Of the 230 patients treated with Noxafil® delayed-release tablets, 38 (17%) were greater than 65 years of age. Of the 288 patients randomized to Posaconazole injection/ Noxafil® delayed-release tablets in the Aspergillosis Treatment Study, 85 (29%) were ≥65 years of age.
No overall differences in the pharmacokinetics and safety were observed between elderly and young subjects during clinical trials, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Posaconazole Injection should be avoided in patients with moderate or severe renal impairment (eGFR <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Posaconazole injection. In patients with moderate or severe renal impairment (eGFR <50 mL/min), receiving the Posaconazole injection, accumulation of the intravenous vehicle, SBECD, is expected to occur. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral Posaconazole therapy [see Dosage and Administration (2.9) and Warnings and Precautions (5.5)].
8.7 Hepatic Impairment
It is recommended that no dose adjustment of Posaconazole injection is needed in patients with mild to severe hepatic impairment (Child-Pugh Class A, B, or C) [see Dosage and Administration (2) and Warnings and Precautions (5.4)]. However, a specific study has not been conducted with Posaconazole injection.
8.8 Gender
The pharmacokinetics of posaconazole are comparable in males and females. No adjustment in the dosage of Posaconazole is necessary based on gender.
-
10 OVERDOSAGE
There is no experience with overdosage of Posaconazole injection.
During the clinical trials, some patients received Noxafil® oral suspension up to 1600 mg/day with no adverse reactions noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg twice daily Noxafil® oral suspension for 3 days. No related adverse reactions were noted by the investigator.
Posaconazole is not removed by hemodialysis.
-
11 DESCRIPTION
Posaconazole is an azole antifungal agent. Posaconazole is available as injection solution to be diluted before intravenous administration.
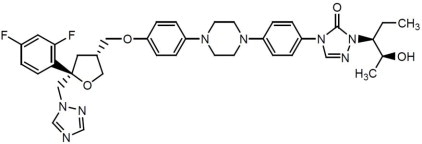
Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (2,4-difluorophenyl)tetrahydro-5 (1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2 hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one with an empirical formula of C37H42F2N8O4 and a molecular weight of 700.8. The chemical structure is:
Posaconazole is a white powder with a low aqueous solubility.
Posaconazole Injection is available as a clear colorless to yellow, sterile liquid essentially free of foreign matter. Each vial contains 300 mg of posaconazole and the following inactive ingredients: 6.68 g Betadex Sulfobutyl Ether Sodium (SBECD), 0.0033 g edetate disodium, hydrochloric acid and sodium hydroxide to adjust the pH to 2.6, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Posaconazole is an azole antifungal agent [see Clinical Pharmacology (12.4)].
12.2 Pharmacodynamics
Exposure Response Relationship Prophylaxis: In clinical studies of neutropenic patients who were receiving cytotoxic chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) or hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD), a wide range of plasma exposures to posaconazole was noted following administration of Noxafil® oral suspension. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cavg) and prophylactic efficacy (Table 17). A lower Cavg may be associated with an increased risk of treatment failure, defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections.
Table 17: Noxafil® Oral Suspension Exposure Analysis (Cavg) in Prophylaxis Trials Cavg = the average posaconazole concentration when measured at steady state
* Neutropenic patients who were receiving cytotoxic chemotherapy for AML or MDS
† HSCT recipients with GVHD
‡ Defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections
Prophylaxis in AML/MDS*Prophylaxis in GVHD† Cavg Range (ng/mL) Treatment Failure‡ (%) Cavg Range (ng/mL) Treatment Failure‡ (%)
Quartile 1
90-322
54.7
22-557
44.4
Quartile 2
322-490
37.0
557-915
20.6
Quartile 3
490-734
46.8
915-1563
17.5
Quartile 4
734-2200
27.8
1563-3650
17.5Exposure Response Relationship Treatment of Invasive Aspergillosis:
Across a range of posaconazole plasma minimum concentrations (Cmin, range: 244 to 5663 ng/mL) following administration of Posaconazole injection and Noxafil® delayed-release tablets in patients treated for invasive aspergillosis in Aspergillosis Treatment Study, there was no association between posaconazole Cmin and treatment efficacy [see Clinical Pharmacology (12.3) and Clinical Studies (14.1)]. Similarly, across a range of population pharmacokinetic model-predicted steady-state plasma average concentrations (Cavg, range: 589 to 6315 ng/mL), there was no association between posaconazole Cavg and treatment efficacy.
12.3 Pharmacokinetics
Posaconazole Injection
Posaconazole injection exhibits dose proportional pharmacokinetics after single doses between 200 and 300 mg in healthy volunteers and patients. The mean pharmacokinetic parameters after single doses with Posaconazole injection in healthy volunteers and patients are shown in Table 18.
Table 18: Summary of Mean Pharmacokinetic Parameters (%CV) in Healthy Volunteers (30-minute infusion via peripheral venous line) and Patients (90 minute infusion via central venous line) after Dosing with Posaconazole Injection on Day 1 AUC0-∞ = Area under the plasma concentration-time curve from time zero to infinity; AUC0-12 = Area under the plasma concentration- time curve from time zero to 12 hr after the first dose on Day 1; Cmax = maximum observed concentration; t½ = terminal phase half- life; CL = total body clearance; N/D = Not Determined
Dose (mg) n AUC0-∞
(ng·hr/mL)AUC0-12
(ng·hr/mL)Cmax (ng/mL) t1/2 (hr) CL
(L/hr)Healthy Volunteers 200 9 35400 (50) 8840 (20) 2250 (29) 23.6 (23) 6.5 (32) 300 9 46400 (26) 13000 (13) 2840 (30) 24.6 (20) 6.9 (27)
Patients200 30 N/D 5570 (32) 954 (44) N/D N/D 300 22 N/D 8240 (26) 1590 (62) N/D N/D Table 19 displays the pharmacokinetic parameters of posaconazole in patients following administration of Posaconazole injection 300 mg taken once a day for 10 or 14 days following twice daily dosing on Day 1.
Table 19: Arithmetic Mean (%CV) of PK Parameters in Serial PK-Evaluable Patients Following Dosing of Posaconazole Injection (300 mg)* AUC0-24 = area under the concentration-time curve over the dosing interval (i.e. 24 hours); Cav= time-averaged concentrations (i.e., AUC0-24h/24hr);
Cmin = POS trough level immediately before a subject received the dose of POS on the day specified in the protocol; Cmax = observed maximum plasma concentration; CV = coefficient of variation, expressed as a percent (%); Day = study day on treatment; Tmax = time of observed maximum plasma concentration.
* 300 mg dose administered over 90 minutes once a day following twice daily dosing on Day 1
† Median (minimum-maximum)
Day N Cmax (ng/mL) Tmax†
(hr)AUC0-24
(ng*hr/mL)Cav (ng/mL) Cmin (ng/mL) 10/14 49 3280 (74) 1.5 (0.98-4.0) 36100 (35) 1500 (35) 1090 (44) Distribution:
The mean volume of distribution of posaconazole after intravenous solution administration was 261 L and ranged from 226-295 L between studies and dose levels.
Posaconazole is highly bound to human plasma proteins (>98%), predominantly to albumin.
Metabolism:
Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Posaconazole is primarily metabolized via UDP glucuronidation (phase 2 enzymes) and is a substrate for p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. A summary of drugs studied clinically with the oral suspension or an early tablet formulation, which affect posaconazole concentrations, is provided in Table 27.
Table 27: Summary of the Effect of Coadministered Drugs on Posaconazole in Healthy Volunteers * Ratio Estimate is the ratio of coadministered drug plus Posaconazole to Posaconazole alone for Cmax or AUC.
† The tablet refers to a non-commercial tablet formulation without polymer.
Coadministered Drug (Postulated Mechanism of Interaction)
Coadministered Drug Dose/Schedule
Posaconazole Dose/Schedule
Effect on Bioavailability of PosaconazoleChange in Mean Cmax
(ratio estimate*; 90% CI of the ratio estimate)Change in Mean AUC
(ratio estimate*; 90% CI of the ratio estimate)Efavirenz 400 mg once daily × 10 and 400 mg (oral ↓45% ↓ 50% (UDP-G Induction) 20 days suspension) twice daily × 10 and 20 days (0.55; 0.47-0.66) (0.50; 0.43-0.60) Fosamprenavir 700 mg twice daily x
10 days200 mg once daily on the 1st ↓21% ↓23% (unknown mechanism) day, 200 mg twice daily on
the 2nd day, then 4000.79 (0.71-0.89) 0.77 (0.68-0.87) mg twice daily x 8 Days Rifabutin
(UDP-G Induction)300 mg once daily x
17 days200 mg (tablets) once
daily × 10 days†↓ 43%
(0.57; 0.43-0.75)↓ 49%
(0.51; 0.37-0.71)Phenytoin
(UDP-G Induction)200 mg once daily x
10 days200 mg (tablets) once
daily × 10 days†↓ 41%
(0.59; 0.44-0.79)↓ 50%
(0.50; 0.36-0.71)In vitro studies with human hepatic microsomes and clinical studies indicate that posaconazole is an inhibitor primarily of CYP3A4. A clinical study in healthy volunteers also indicates that posaconazole is a strong CYP3A4 inhibitor as evidenced by a >5-fold increase in midazolam AUC. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole. A summary of the drugs studied clinically, for which plasma concentrations were affected by posaconazole, is provided in Table 28 [see Contraindications (4) and Drug Interactions (7.1) including recommendations].
Table 28: Summary of the Effect of Posaconazole on Coadministered Drugs in Healthy Adult Volunteers and Patients * Ratio Estimate is the ratio of coadministered drug plus Posaconazole to coadministered drug alone for Cmax or AUC.
† The tablet refers to a non-commercial tablet formulation without polymer.
‡ The mean terminal half-life of midazolam was increased from 3 hours to 7 to 11 hours during coadministration with Posaconazole.
Coadministered Drug (Postulated Mechanism of Interaction is Inhibition of CYP3A4 by
posaconazole)
Coadministered Drug Dose/Schedule
Posaconazole Dose/ ScheduleEffect on Bioavailability of Coadministered
DrugsChange in Mean Cmax
(ratio estimate*; 90% CI of the ratio estimate)
Change in Mean AUC (ratio estimate*; 90% CI of the ratio estimate)Sirolimus 2-mg single oral dose 400 mg (oral suspension) twice daily x 16 days ↑ 572%
(6.72; 5.62-8.03)↑ 788%
(8.88; 7.26-10.9)Cyclosporine Stable maintenance dose in heart transplant recipients 200 mg (tablets) once daily x 10 days† ↑ cyclosporine whole blood trough concentrations
Cyclosporine dose reductions of up to 29% were requiredTacrolimus 0.05-mg/kg single oral dose 400 mg (oral suspension) twice daily × 7 days ↑ 121%
(2.21; 2.01-2.42)↑ 358%
(4.58; 4.03-5.19)Simvastatin 40-mg single oral dose 100 mg (oral suspension) Simvastatin Simvastatin once daily x 13 days ↑ 841% ↑ 931% (9.41, 7.13-12.44) (10.31, 8.40-12.67) Simvastatin Acid Simvastatin Acid ↑ 817% ↑634% (9.17, 7.36-11.43) (7.34, 5.82-9.25)
200 mg (oral suspension)
Simvastatin
Simvastatinonce daily x 13 days ↑ 1041% ↑ 960% (11.41, 7.99-16.29) (10.60, 8.63-13.02) Simvastatin Acid Simvastatin Acid ↑851% ↑748% (9.51, 8.15-11.10) (8.48, 7.04-10.23) Midazolam 0.4-mg single 200 mg (oral suspension) ↑ 30% ↑ 362% intravenous dose‡ twice daily x 7 days (1.3; 1.13-1.48) (4.62; 4.02-5.3) 0.4-mg single 400 mg (oral suspension) ↑62% ↑524% intravenous dose‡ twice daily x 7 days (1.62; 1.41-1.86) (6.24; 5.43-7.16)
2-mg single oral dose‡200 mg (oral suspension) once daily x 7 days ↑ 169%
(2.69; 2.46-2.93)↑ 470%
(5.70; 4.82-6.74)
2-mg single oral dose‡400 mg (oral suspension) twice daily x 7 days ↑ 138%
(2.38; 2.13-2.66)↑ 397%
(4.97; 4.46-5.54)Rifabutin 300 mg once daily x 17 days 200 mg (tablets) once daily
× 10 days†↑ 31%
(1.31; 1.10-1.57)↑ 72%
(1.72;1.51-1.95)Phenytoin 200 mg once daily PO x 10
days200 mg (tablets) once daily
x 10 days†↑ 16%
(1.16; 0.85-1.57)↑ 16%
(1.16; 0.84-1.59)Ritonavir 100 mg once daily x 14 days 400 mg (oral suspension) twice daily x 7 days ↑ 49%
(1.49; 1.04-2.15)↑ 80%
(1.8;1.39-2.31)Atazanavir 300 mg once daily x 14 days 400 mg (oral suspension) ↑ 155% ↑ 268% twice daily x 7 days (2.55; 1.89-3.45) (3.68; 2.89-4.70) Atazanavir/ ritonavir boosted 300 mg/100 mg once daily 400 mg (oral suspension) ↑ 53% ↑ 146% regimen x 14 days twice daily x 7 days (1.53; 1.13-2.07) (2.46; 1.93-3.13) Additional clinical studies demonstrated that no clinically significant effects on zidovudine, lamivudine, indinavir, or caffeine were observed when administered with Posaconazole 200 mg once daily; therefore, no dose adjustments are required for these coadministered drugs when coadministered with Posaconazole 200 mg once daily.
Excretion:
Following administration of Noxafil® oral suspension, posaconazole is predominantly eliminated in the feces (71% of the radiolabeled dose up to 120 hours) with the major component eliminated as parent drug (66% of the radiolabeled dose). Renal clearance is a minor elimination pathway, with 13% of the radiolabeled dose excreted in urine up to 120 hours (<0.2% of the radiolabeled dose is parent drug).
Posaconazole injection is eliminated with a mean terminal half-life (t½) of 27 hours and a total body clearance (CL) of 7.3 L/h.
Specific Populations
No clinically significant differences in the pharmacokinetics of posaconazole were observed based on age, sex, renal impairment, and indication (prophylaxis or treatment).
Race/Ethnicity:
In a population pharmacokinetic analysis of posaconazole, AUC was found to be 25% higher in Chinese patients relative to patients from other races/ethnicities. This higher exposure is not expected to be clinically relevant given the expected variability in posaconazole exposure.
Patients Weighing More Than 120 kg:
Weight has a clinically significant effect on posaconazole clearance. Relative to 70 kg patients, the Cavg is decreased by 25% in patients greater than 120 kg. Patients administered Posaconazole weighing more than 120 kg may be at higher risk for lower posaconazole plasma concentrations compared to lower weight patients [see Use in Specific Populations (8.10)].
Pediatric Patients
The mean pharmacokinetic parameters after multiple-dose administration of Posaconazole injection and Noxafil® PowderMix for delayed-release oral suspension in neutropenic pediatric patients 2 to less than 18 years of age are shown in Table 29. Patients were enrolled into 2 age groups and received Posaconazole injection and Noxafil® PowderMix for delayed-release oral suspension doses at 6 mg/kg (0.6 to 1 times the recommended dose) with a maximum 300 mg dose once daily (twice daily on Day 1) [see Adverse Reactions (6.1)].
Table 29: Summary of Steady-State Geometric Mean Pharmacokinetic Parameters (% Geometric CV) After Multiple Dosing with Posaconazole Injection and Noxafil® PowderMix for Delayed-Release Oral Suspension 6 mg/kg* in Pediatric Patients with Neutropenia or Expected Neutropenia IV= Posaconazole injection; PFS= Noxafil® PowderMix for delayed-release oral suspension; AUC0-24 = Area under the plasma concentration-time curve from time zero to 24 hr; Cmax = maximum observed concentration; Cmin = minimum observed plasma concentration; Tmax = time of maximum observed concentration; CL /F = apparent total body clearance
* 0.6 to 1 times the recommended dose
† Cav = time-averaged concentrations (i.e., AUC0-24 hr/24hr)
‡ Median (minimum-maximum)
§ Clearance (CL for IV and CL/F for PFS)
Age Group Dose Type N AUC0-24 hr
(ng·hr/mL)Cav† (ng/mL) Cmax (ng/mL) Cmin (ng/mL) T ‡
max
(hr)CL/F§ (L/hr) 2 to <7 IV 17 31100 1300 3060 626 1.75 3.27 years (48.9) (48.9) (54.1) (104.8) (1.57-1.83) (49.3) PFS 7 23000 960 (47.3) 1510 542 4.00 4.60 (47.3) (43.4) (68.8) (2.17-7.92) (35.2) 7 to 17 years IV 24 44200
(41.5)1840
(41.5)3340
(39.4)1160
(60.4)1.77
(1.33-6.00)4.76
(55.7)PFS 12 25000
(184.3)1040
(184.3)1370
(178.5)713
(300.6)2.78
(0.00-4.00)8.39
(190.3)Based on a population pharmacokinetic model evaluating posaconazole pharmacokinetics and predicting exposures in pediatric patients, the exposure of steady-state posaconazole average concentration greater than or equal to 700 ng/mL in approximately 90% of patients is attained with the recommended dose of Posaconazole injection and Noxafil® PowderMix for delayed-release oral suspension.
The population pharmacokinetic analysis of posaconazole in pediatric patients suggests that age, sex, renal impairment and ethnicity have no clinically meaningful effect on the pharmacokinetics of posaconazole.
A total of 12 patients 13 to 17 years of age received 600 mg/day (200 mg three times a day) of Noxafil® oral suspension for prophylaxis of invasive fungal infections. Based on pharmacokinetic data in 10 of these pediatric patients, the mean steady-state Cav was similar between these patients and adults (≥18 years of age). In a study of 136 neutropenic pediatric patients 11 months to less than 18 years treated with Noxafil® oral suspension, the exposure target of steady-state posaconazole Cavg between 500 ng/mL and less than 2500 ng/mL was attained in approximately 50% of patients instead of the pre-specified 90% of patients.
12.4 Microbiology
Mechanism of Action:
Posaconazole blocks the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14α-demethylase responsible for the conversion of lanosterol to ergosterol in the fungal cell membrane. This results in an accumulation of methylated sterol precursors and a depletion of ergosterol within the cell membrane thus weakening the structure and function of the fungal cell membrane. This may be responsible for the antifungal activity of posaconazole.
Resistance:
Clinical isolates of Candida albicans and Candida glabrata with decreased susceptibility to posaconazole were observed in oral swish samples taken during prophylaxis with posaconazole and fluconazole, suggesting a potential for development of resistance. These isolates also showed reduced susceptibility to other azoles, suggesting cross-resistance between azoles. The clinical significance of this finding is not known.
Antimicrobial Activity:
Posaconazole has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No drug-related neoplasms were recorded in rats or mice treated with posaconazole for 2 years at doses higher than the clinical dose. In a 2-year carcinogenicity study, rats were given posaconazole orally at doses up to 20 mg/kg (females), or 30 mg/kg (males). These doses are equivalent to 3.9- or 3.5-times the exposure achieved with a 400-mg twice daily oral suspension regimen, respectively, based on steady-state AUC in healthy volunteers administered a high-fat meal (400-mg twice daily oral suspension regimen). In the mouse study, mice were treated at oral doses up to 60 mg/kg/day or 4.8-times the exposure achieved with a 400-mg twice daily oral suspension regimen.
Mutagenesis
Posaconazole was not genotoxic or clastogenic when evaluated in bacterial mutagenicity (Ames), a chromosome aberration study in human peripheral blood lymphocytes, a Chinese hamster ovary cell mutagenicity study, and a mouse bone marrow micronucleus study.
Impairment of Fertility
Posaconazole had no effect on fertility of male rats at a dose up to 180 mg/kg (1.7 x the 400-mg twice daily oral suspension regimen based on steady-state plasma concentrations in healthy volunteers) or female rats at a dose up to 45 mg/kg (2.2 x the 400-mg twice daily oral suspension regimen).
13.2 Animal Toxicology and/or Pharmacology
In a nonclinical study using intravenous administration of posaconazole in very young dogs (dosed from 2 to 8 weeks of age), an increase in the incidence of brain ventricle enlargement was observed in treated animals as compared with concurrent control animals. No difference in the incidence of brain ventricle enlargement between control and treated animals was observed following the subsequent 5-month treatment-free period. There were no neurologic, behavioral or developmental abnormalities in the dogs with this finding, and a similar brain finding was not seen with oral posaconazole administration to juvenile dogs (4 days to 9 months of age). There were no drug-related increases in the incidence of brain ventricle enlargement when treated and control animals were compared in a separate study of 10-week old dogs dosed with intravenous posaconazole for 13 weeks with a 9-week recovery period or a follow-up study of 31-week old dogs dosed for 3 months.
-
14 CLINICAL STUDIES
14.1 Treatment of Invasive Aspergillosis with Posaconazole Injection and Noxafil® Delayed-Release Tablets
Aspergillosis Treatment Study (NCT01782131) was a randomized, double-blind, controlled trial which evaluated the safety and efficacy of Posaconazole injection and Noxafil® delayed-release tablets versus voriconazole for primary treatment of invasive fungal disease caused by Aspergillus species. Eligible patients had proven, probable, or possible invasive fungal infections per the European Organization for Research and Treatment of Cancer/Mycoses Study Group, EORTC/MSG criteria. Patients were stratified by risk for mortality or poor outcome where high risk included a history of allogeneic bone marrow transplant, liver transplant, or relapsed leukemia undergoing salvage chemotherapy. The median age of patients was 57 years (range 14-91 years), with 27.8% of patients aged ≥65 years; 5 patients were pediatric patients 14-16 years of age, of whom 3 were treated with Posaconazole and 2 with voriconazole. The majority of patients were male (59.8%) and white (67.1%). With regard to risk factors for invasive aspergillosis, approximately two- thirds of the patients in the study had a recent history of neutropenia, while approximately 20% with a history of an allogeneic stem cell transplant. Over 80% of subjects in each treatment group had infection limited to the lower respiratory tract (primarily lung), while approximately 11% to 13% also had infection in another organ. Invasive aspergillosis was proven or probable in 58.1% of patients as classified by independent adjudicators blinded to study treatment assignment. At least one Aspergillus species was identified in 21% of the patients; A. fumigatus and A. flavus were the most common pathogens identified.
Patients randomized to receive Posaconazole were given a dose of 300 mg once daily (twice daily on Day 1) IV or tablet. Patients randomized to receive voriconazole were given a dose of 6 mg/kg twice daily Day 1 followed by 4 mg/kg twice daily IV, or oral 300 mg twice daily Day 1 followed by 200 mg twice daily. The recommended initial route of administration was IV; however, patients could begin oral therapy if clinically stable and able to tolerate oral dosing. The transition from IV to oral therapy occurred when the patient was clinically stable. The protocol recommended duration of therapy was 84 days with a maximum allowed duration of 98 days. Median treatment duration was 67 days for Posaconazole patients and 64 days for voriconazole patients. Overall, 55% to 60% of patients began treatment with the IV formulation with a median duration of 9 days for the initial IV dosing.
The Intent to Treat (ITT) population included all patients randomized and receiving at least one dose of study treatment. All-cause mortality through Day 42 in the overall population (ITT) was 15.3% for Posaconazole patients compared to 20.6% for voriconazole patients for an adjusted treatment difference of -5.3% with a 95% confidence interval of -11.6 to 1.0%. Consistent results were seen in patients with proven or probable invasive aspergillosis per EORTC criteria (see Table 30).
Table 30: Posaconazole Injection and Noxafil® Delayed-Release Tablets Invasive Aspergillosis Treatment Study: All-Cause Mortality Through Day 42 * Adjusted treatment difference based on Miettinen and Nurminen's method stratified by randomization factor (risk for mortality/poor outcome), using Cochran-Mantel-Haenszel weighting scheme.
Posaconazole Injection and Delayed- Release Tablets Voriconazole Population N n (%) N n (%) Difference* (95% CI) Intent to Treat 288 44 (15.3) 287 59 (20.6) -5.3 (-11.6, 1.0) Proven/Probable Invasive Aspergillosis 163 31 (19.0) 171 32 (18.7) 0.3 (-8.2, 8.8) Global clinical response at Week 6 was assessed by a blinded, independent adjudication committee based upon prespecified clinical, radiologic, and mycologic criteria. In the subgroup of patients with proven or probable invasive aspergillosis per EORTC criteria, the global clinical response of success (complete or partial response) at Week 6 was seen in 44.8% for Posaconazole-treated patients compared to 45.6% for voriconazole-treated patients (see Table 31).
Table 31: Posaconazole Injection and Noxafil® Delayed-Release Tablets Invasive Aspergillosis Treatment Study: Successful Global Clinical Response* at Week 6 * Successful Global Clinical Response was defined as survival with a partial or complete response
† Adjusted treatment difference based on Miettinen and Nurminen's method stratified by randomization factor (risk for mortality/poor outcome), using Cochran-Mantel-Haenszel weighting scheme.
Posaconazole Voriconazole Population N Success N Success Difference† (95% CI) Proven/Probable Invasive Aspergillosis 163 73 (44.8) 171 78 (45.6) -0.6 (-11.2, 10.1) -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Injection
Posaconazole Injection is available in Type I glass vials closed with chlorobutyl rubber stopper and aluminum seal.
Product Code Unit of Sale Strength Each 685017 NDC: 63323-685-17
Individually packaged300 mg per 16.7 mL of solution (18 mg of Posaconazole per mL) 300 mg per 16.7 mL (18 mg per mL)
Single-Dose Vial16.2 Storage and Handling
Posaconazole Injection
Posaconazole injection vial should be stored refrigerated at 2° to 8°C (36° to 46°F). Storage conditions for the diluted solution are presented in another section of the prescribing information [see Dosage and Administration (2.4)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions
Advise patients to inform their physician immediately if they:
- develop severe diarrhea or vomiting.
- are currently taking drugs that are known to prolong the QTc interval and are metabolized through CYP3A4.
- are currently taking a cyclosporine or tacrolimus, or they notice swelling in an arm or leg or shortness of breath.
- are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of posaconazole.
Serious and Potentially Serious Adverse Reactions
Advise patients to inform their physician immediately if they:
- notice a change in heart rate or heart rhythm or have a heart condition or circulatory disease. Posaconazole can be administered with caution to patients with potentially proarrhythmic conditions.
- are pregnant, plan to become pregnant, or are nursing.
- have liver disease or develop itching, nausea or vomiting, their eyes or skin turn yellow, they feel more tired than usual or feel like they have the flu.
- have ever had an allergic reaction to other antifungal medicines such as ketoconazole, fluconazole, itraconazole, or voriconazole.
The container closure is not made with natural rubber latex.
Manufactured by:
Lake Zurich, IL 60047
www.fresenius-kabi.com/us
451561A -
PATIENT PACKAGE INSERT
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – Posaconazole Injection Vial Label
NDC: 63323-685-17
Posaconazole Injection
300 mg per 16.7 mL
(18 mg per mL)
For Intravenous Use Only
Requires further dilution prior to infusion.
Discard Unused Portion
Single-Dose Vial Rx only
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY – Posaconazole Injection Carton Panel
NDC: 63323-685-17 685017
Posaconazole Injection
300 mg per 16.7 mL
(18 mg per mL)
For Intravenous Use Only
Requires further dilution prior to infusion.
Rx only
Sterile Single-Dose Vial
Discard Unused Portion
-
INGREDIENTS AND APPEARANCE
POSACONAZOLE
posaconazole injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-685 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Posaconazole (UNII: 6TK1G07BHZ) (Posaconazole - UNII:6TK1G07BHZ) Posaconazole 18 mg in 1 mL Inactive Ingredients Ingredient Name Strength betadex sulfobutyl ether sodium (UNII: 2PP9364507) edetate disodium (UNII: 7FLD91C86K) hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-685-17 1 in 1 CARTON 12/26/2023 1 16.7 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209983 12/26/2023 Labeler - Fresensius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresensius Kabi USA, LLC 840771732 MANUFACTURE(63323-685) , ANALYSIS(63323-685)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.