Diclofenac sodium by Pharmaceutica North America, Inc DICLOFENAC SODIUM gel
Diclofenac sodium by
Drug Labeling and Warnings
Diclofenac sodium by is a Prescription medication manufactured, distributed, or labeled by Pharmaceutica North America, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
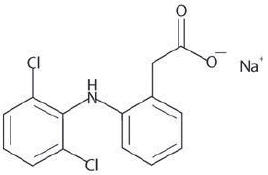
Diclofenac Sodium Gel, 3%, contains the active ingredient, diclofenac sodium, in a clear, transparent, colorless to slightly yellow gel base. Diclofenac sodium is a white to slightly yellow crystalline powder. It is freely soluble in methanol, soluble in ethanol, sparingly soluble in water, slightly soluble in acetone, and partially insoluble in ether. The chemical name for diclofenac sodium is:
Sodium [o-(2,6-dichloranilino) phenyl] acetate
Diclofenac sodium has a molecular weight of 318.13.
The CAS number is CAS-15307-79-6. The structural formula is represented below:
Diclofenac Sodium Gel, 3% also contains benzyl alcohol, hyaluronate sodium, polyethylene glycol monomethyl ether, and purified water.
1 g of Diclofenac Sodium Gel contains 30 mg of the active substance, diclofenac sodium.
-
CLINICAL PHARMACOLOGY
The mechanism of action of diclofenac sodium in the treatment of actinic keratoses (AK) is unknown. The contribution to efficacy of individual components of the vehicle has not been established.
Pharmacokinetics
Absorption
When diclofenac sodium gel is applied topically, diclofenac is absorbed into the epidermis. In a study in patients with compromised skin (mainly atopic dermatitis and other dermatitic conditions) of the hands, arms or face, approximately 10% of the applied dose (2 grams of 3% gel over 100 cm2) of diclofenac was absorbed systemically in both normal and compromised epidermis after seven days, with four times daily applications.
After topical application of 2 g diclofenac sodium gel three times daily for six days to the calf of the leg in healthy subjects, diclofenac could be detected in plasma. Mean bioavailability parameters were AUC0-t 9±19 ng/hr/mL (mean±SD) with a Cmax of 4±5 ng/mL and a Tmax of 4.5±8 hours. In comparison, a single oral 75 mg dose of diclofenac (Voltaren®)1 produced an AUC of 1600 ng/hr/mL. Therefore, the systemic bioavailability after topical application of diclofenac sodium gel is lower than after oral dosing.
Comparative bioavailability studies have not been conducted between available diclofenac topical products (gels containing 1 to 3% diclofenac) which have different dosing regimens. A cross-study evaluation of the data indicates that diclofenac is more bioavailable when applied to diseased skin and less bioavailable when applied to intact skin.
Blood drawn at the end of treatment from 60 patients with AK lesions treated with diclofenac sodium gel in three adequate and well-controlled clinical trials was assayed for diclofenac levels. Each patient was administered 0.5 g of diclofenac sodium gel twice a day for up to 105 days. There were up to three 5 cm × 5 cm treatment sites per patient on the face, forehead, hands, forearm, and scalp. Serum concentrations of diclofenac were, on average, at or below 20 ng/mL. These data indicate that systemic absorption of diclofenac in patients treated topically with diclofenac sodium gel is much lower than that occurring after oral daily dosing of diclofenac sodium.
No information is available on the absorption of diclofenac when diclofenac sodium gel is used under occlusion.
- 1 Voltaren® is a registered trademark of Novartis.
Distribution
Diclofenac binds tightly to serum albumin. The volume of distribution of diclofenac following oral administration is approximately 550 mL/kg.
Metabolism
Biotransformation of diclofenac following oral administration involves conjugation at the carboxyl group of the side chain or single or multiple hydroxylations resulting in several phenolic metabolites, most of which are converted to glucuronide conjugates. Two of these phenolic metabolites are biologically active, however to a much smaller extent than diclofenac. Metabolism of diclofenac following topical administration is thought to be similar to that after oral administration. The small amounts of diclofenac and its metabolites appearing in the plasma following topical administration makes the quantification of specific metabolites imprecise.
- INDICATIONS AND USAGE
-
CLINICAL STUDIES
Clinical trials were conducted involving a total of 427 patients (213 treated with diclofenac sodium gel and 214 with a gel vehicle). Each patient had no fewer than five AK lesions in a major body area, which was defined as one of five 5 cm × 5 cm regions: scalp, forehead, face, forearm and hand. Up to three major body areas were studied in any patient. All patients were 18 years of age or older (male and female) with no clinically significant medical problems outside of the AK lesions and had undergone a 60-day washout period from disallowed medications (masoprocol, 5-fluorouracil, cyclosporine, retinoids, trichloroacetic acid/lactic acid/peel, 50% glycolic acid peel) and hyaluronan-containing cosmetics. Patients were excluded from participation for reasons of known or suspected hypersensitivity to any diclofenac sodium gel ingredient, pregnancy, allergies to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs), or other dermatological conditions which might affect the absorption of the study medication. Application of dermatologic products such as sunscreens, cosmetics, and other drug products was not permitted. Patients were instructed to apply a small amount of diclofenac sodium gel (approximately 0.5 g) onto the affected skin, using their fingers, and gently smoothing the gel over the lesion. In addition, all patients were instructed to avoid sun exposure. Complete clearing of the AK lesions 30 days after completion of treatment was the primary efficacy variable. No long-term patient follow-ups, after the 30-day assessments, were performed for the detection of recurrence.
Complete Clearance of Actinic Keratosis Lesions 30 Days Post-Treatment (all locations) Diclofenac Sodium Gel Vehicle p-value Study 1 90 days treatment 27/58 (47%) 11/59 (19%) <0.001 Study 2 90 days treatment 18/53 (34%) 10/55 (18%) 0.061 Study 3 60 days treatment 15/48 (31%) 5/49 (10%) 0.021 30 days treatment 7/49 (14%) 2/49 (4%) 0.221 Complete Clearance of Actinic Keratosis Lesions 30 Days Post-Treatment (by location) Scalp Forehead Face Arm/Forearm Back of Hand Study 1 90 days treatment Diclofenac Sodium Gel 1/4 (25%) 17/30 (57%) 9/17 (53%) 4/12 (33%) 6/16 (38%) Vehicle 3/9 (33%) 8/24 (33%) 5/17 (29%) 4/12 (33%) 0/14 (0) p-value 0.7646 0.0908 0.1682 1.000 0.0650 Study 2 90 days treatment Diclofenac Sodium Gel 2/6 (33%) 9/19 (47%) 4/5 (80%) 5/8 (63%) 1/17 (6%) Vehicle 0/4 (0) 6/22 (27%) 2/8 (25%) 0/5 (0) 3/16 (19%) p-value 0.4235 0.1870 0.0727 0.0888 0.2818 Study 3 60 days treatment Diclofenac Sodium Gel 3/7 (43%) 13/31 (42%) 10/19 (53%) 0/1 (0) 2/8 (25%) Vehicle 0/6 (0) 5/36 (14%) 2/13 (15%) 0/2 (0) 1/9 (11%) p-value 0.2271 0.0153 0.0433 - -0.4637 30 days treatment Diclofenac Sodium Gel 2/5 (40%) 4/29 (14%) 3/14 (21%) 0/0 (0) 0/9 (0) Vehicle 0/5 (0) 2/29 (7%) 2/18 (11%) 0/1 (0) 1/9 (11%) p-value 0.2299 0.3748 0.4322 - -0.6521 All data combined Diclofenac Sodium Gel 8/22 (36%) 43/109 (39%) 26/55 (47%) 9/21 (43%) 9/50 (18%) Vehicle 3/24 (13%) 21/111 (19%) 11/56 (20%) 4/20 (20%) 5/48 (10%) p-value 0.0903 0.0013 0.0016 0.2043 0.3662 - CONTRAINDICATIONS
-
WARNINGS
As with other NSAIDs, anaphylactoid reactions may occur in patients without prior exposure to diclofenac. Diclofenac sodium should be given with caution to patients with the aspirin triad. The triad typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs.
-
PRECAUTIONS
General
Diclofenac Sodium Gel should be used with caution in patients with active gastrointestinal ulceration or bleeding and severe renal or hepatic impairments. Diclofenac sodium gel should not be applied to open skin wounds, infections, or exfoliative dermatitis. It should not be allowed to come in contact with the eyes.
The safety of the concomitant use of sunscreens, cosmetics or other topical medications and diclofenac sodium gel is unknown.
Information for Patients
In clinical studies, localized dermal side effects such as contact dermatitis, exfoliation, dry skin and rash were found in patients treated with diclofenac sodium gel at a higher incidence than in those with placebo.
Patients should understand the importance of monitoring and follow-up evaluation, the signs and symptoms of dermal adverse reactions, and the possibility of irritant or allergic contact dermatitis. If severe dermal reactions occur, treatment with diclofenac sodium gel may be interrupted until the condition subsides. Exposure to sunlight and the use of sunlamps should be avoided.
Safety and efficacy of the use of diclofenac sodium gel together with other dermal products, including cosmetics, sunscreens, and other topical medications on the area being treated, have not been studied.
Drug Interactions
Specific interaction studies between diclofenac sodium gel and other topical or oral agents were not performed.
Oral Nonsteroidal Anti-Inflammatory Drugs
Although low, there is systemic exposure to diclofenac following labeled use of diclofenac sodium gel. Therefore, concomitant administration of diclofenac sodium gel with oral NSAIDs or aspirin may result in increased NSAID adverse effects.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There did not appear to be any increase in drug-related neoplasms following daily topical applications of diclofenac sodium gel for 2 years at concentrations up to 0.035% diclofenac sodium and 2.5% hyaluronate sodium in albino mice. (Note: diclofenac sodium gel contains 3% diclofenac sodium.) When administered orally for 2 years, diclofenac showed no evidence of carcinogenic potential in rats given diclofenac sodium at up to 2 mg/kg/day (3 times the estimated systemic human exposure2), or in mice given diclofenac sodium at up to 0.3 mg/kg/day in males and 1 mg/kg/day in females (25% and 83%, respectively, of the estimated systemic human exposure).
A photococarcinogenicity study with up to 0.035% diclofenac in the diclofenac sodium vehicle gel was conducted in hairless mice at topical doses up to 2.8 mg/ kg/day. Median tumor onset was earlier in the 0.035% group (diclofenac sodium gel contains 3% diclofenac sodium).
Diclofenac was not genotoxic in in vitro point mutation assays in mammalian mouse lymphoma cells and Ames microbial test systems, or when tested in mammalian in vivo assays including dominant lethal and male germinal epithelial chromosomal studies in mice, and nucleus anomaly and chromosomal aberration studies in Chinese hamsters. It was also negative in the transformation assay utilizing BALB/3T3 mouse embryo cells.
Fertility studies have not been conducted with diclofenac sodium gel. Diclofenac sodium showed no evidence of impairment of fertility after oral treatment with 4 mg/kg/day (7 times the estimated systemic human exposure) in male or female rats.
- 2 Based on body surface area and assuming 10% bioavailability following topical application of 2 g diclofenac sodium gel per day (1 mg/kg diclofenac sodium).
Pregnancy
Teratogenic Effects
Pregnancy Category B
The safety of diclofenac sodium gel has not been established during pregnancy. However, reproductive studies performed with diclofenac sodium alone at oral doses up to 20 mg/kg/day (15 times the estimated systemic human exposure3) in mice, 10 mg/kg/day (15 times the estimated systemic human exposure) in rats, and 10 mg/kg/day (30 times the estimated systemic human exposure) in rabbits have revealed no evidence of teratogenicity despite the induction of maternal toxicity. In rats, maternally toxic doses were associated with dystocia, prolonged gestation, reduced fetal weights and growth, and reduced fetal survival.
Diclofenac has been shown to cross the placental barrier in mice and rats. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should not be used during pregnancy unless the benefits to the mother justify the potential risk to the fetus. Because of the risk to the fetus resulting in premature closure of the ductus arteriosus, diclofenac should be avoided in late pregnancy.
- 3 Based on body surface area and assuming 10% bioavailability following topical application of 2 g diclofenac sodium gel per day (1 mg/kg diclofenac sodium).
Labor and Delivery
The effects of diclofenac on labor and delivery in pregnant women are unknown. Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), use of diclofenac during late pregnancy should be avoided and, as with other nonsteroidal anti-inflammatory drugs, it is possible that diclofenac may inhibit uterine contractions and delay parturition.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from diclofenac sodium, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Actinic keratoses is not a condition seen within the pediatric population. Diclofenac sodium gel should not be used by children.
Geriatric Use
Of the 211 subjects treated with diclofenac sodium gel in controlled clinical studies, 143 subjects were 65 and over. Of those 143 subjects, 55 subjects were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONS
Of the 423 patients evaluable for safety in adequate and well-controlled trials, 211 were treated with diclofenac sodium gel drug product and 212 were treated with a vehicle gel. Eighty-seven percent (87%) of the diclofenac sodium gel-treated patients (183 patients) and 84% of the vehicle-treated patients (178 patients) experienced one or more adverse events (AEs) during the studies. The majority of these reactions were mild to moderate in severity and resolved upon discontinuation of therapy.
Of the 211 patients treated with diclofenac sodium gel, 172 (82%) experienced AEs involving skin and the application site compared to 160 (75%) vehicle-treated patients. Application site reactions (ASRs) were the most frequent AEs in both diclofenac sodium gel-and vehicle-treated groups. Of note, four reactions, contact dermatitis, rash, dry skin and exfoliation (scaling) were significantly more prevalent in the diclofenac sodium gel group than in the vehicle-treated patients.
Eighteen percent of diclofenac sodium gel-treated patients and 4% of vehicle-treated patients discontinued from the clinical trials due to adverse events (whether considered related to treatment or not). These discontinuations were mainly due to skin irritation or related cutaneous adverse reactions.
Table 1 below presents the AEs reported at an incidence of >1% for patients treated with either diclofenac sodium gel or vehicle (60- and 90-day treatment groups) during the phase 3 studies.
Table 1. Adverse Events Reported (>1% in Any Treatment Group) During Diclofenac Sodium Gel Phase 3 Clinical Trials Incidences for 60-Day and 90-Day Treatments 60-day Treatment 90-day Treatment Diclofenac Sodium
(%) GelVehicle
(%)Diclofenac Sodium
(%) GelVehicle
(%)N=48 N=49 N=114 N=114 BODY AS A WHOLE 21 20 20 18 Abdominal Pain 2 0 1 0 Accidental Injury 0 0 4 2 Allergic Reaction 0 0 1 3 Asthenia 0 0 2 0 Back Pain 4 0 2 2 Chest Pain 2 0 1 0 Chills 0 2 0 0 Flu Syndrome 10 6 1 4 Headache 0 6 7 6 Infection 4 6 4 5 Neck Pain 0 0 2 0 Pain 2 0 2 2 CARDIOVASCULAR SYSTEM 2 4 3 1 Hypertension 2 0 1 0 Migraine 0 2 1 0 Phlebitis 0 2 0 0 DIGESTIVE SYSTEM 4 0 6 8 Constipation 0 0 0 2 Diarrhea 2 0 2 3 Dyspepsia 2 0 3 4 METABOLIC AND NUTRITIONAL DISORDERS 2 8 7 2 Creatine Phosphokinase Increased 0 0 4 1 Creatinine Increased 2 2 0 1 Edema 0 2 0 0 Hypercholesteremia 0 2 1 0 Hyperglycemia 0 2 1 0 SGOT Increased 0 0 3 0 SGPT Increased 0 0 2 0 MUSCULOSKELETAL SYSTEM 4 0 3 4 Arthralgia 2 0 0 2 Arthrosis 2 0 0 0 Myalgia 2 0 3 1 NERVOUS SYSTEM 2 2 2 5 Anxiety 0 2 0 1 Dizziness 0 0 0 4 Hypokinesia 2 0 0 0 RESPIRATORY SYSTEM 8 8 7 6 Asthma 2 0 0 0 Dyspnea 2 0 2 0 Pharyngitis 2 8 2 4 Pneumonia 2 0 0 1 Rhinitis 2 2 2 2 Sinusitis 0 0 2 0 SKIN AND APPENDAGES 75 86 86 71 Acne 0 2 0 1 Application Site Reaction 75 71 84 70 Acne 0 4 1 0 Alopecia 2 0 1 1 Contact Dermatitis 19 4 33 4 Dry Skin 27 12 25 17 Edema 4 0 3 0 Exfoliation 6 4 24 13 Hyperesthesia 0 0 3 1 Pain 15 22 26 30 Paresthesia 8 4 20 20 Photosensitivity Reaction 0 2 3 0 Pruritus 31 59 52 45 Rash 35 20 46 17 Vesiculobullous Rash 0 0 4 1 Contact Dermatitis 2 0 0 0 Dry Skin 0 4 3 0 Herpes Simplex 0 2 0 0 Maculopapular Rash 0 2 0 0 Pain 2 2 1 0 Pruritus 4 6 4 1 Rash 2 10 4 0 Skin Carcinoma 0 6 2 2 Skin Nodule 0 2 0 0 Skin Ulcer 2 0 1 0 SPECIAL SENSES 2 0 4 2 Conjunctivitis 2 0 4 1 Eye Pain 0 2 2 0 UROGENITAL SYSTEM 0 0 4 5 Hematuria 0 0 2 1 OTHER 0 0 0 3 Procedure 0 0 0 3 Skin and Appendages Adverse Events Reported for Diclofenac Sodium Gel at Less Than 1% Incidence in the Phase 3 Studies: skin hypertrophy, paresthesia, seborrhea, urticaria, application site reactions (skin carcinoma, hypertonia, skin hypertrophy lacrimation disorder, maculopapular rash, purpuric rash, vasodilation).
Adverse Reactions Reported for Oral Diclofenac Dosage Form (not topical diclofenac sodium gel):
Body as a Whole: abdominal pain or cramps4, headache4, fluid retention4, abdominal distention4, malaise, swelling of lips and tongue, photosensitivity, anaphylaxis, anaphylactoid reactions, chest pain.
Cardiovascular: hypertension, congestive heart failure, palpitations, flushing, tachycardia, premature ventricular contractions, myocardial infarction, hypotension.
Digestive: diarrhea4, indigestion4, nausea4, constipation4, flatulence4, liver test abnormalities4, PUB4, i.e., peptic ulcer, with or without bleeding and/or perforation, or bleeding without ulcer, vomiting, jaundice, melena, esophageal lesions, aphthous stomatitis, dry mouth and mucous membranes, bloody diarrhea, hepatitis, hepatic necrosis, cirrhosis, hepatorenal syndrome, appetite change, pancreatitis with or without concomitant hepatitis, colitis, intestinal perforation.
Hemic and Lymphatic: hemoglobin decrease, leukopenia, thrombocytopenia, eosinophilia, hemolytic anemia, aplastic anemia, agranulocytosis, purpura, allergic purpura, bruising.
Metabolic and Nutritional Disorders: azotemia, hypoglycemia, weight loss.
Nervous System: dizziness4, insomnia, drowsiness, depression, diplopia, anxiety, irritability, aseptic meningitis, convulsions, paresthesia, memory disturbance, nightmares, tremor, tic, abnormal coordination, disorientation, psychotic reaction.
Respiratory: epistaxis, asthma, laryngeal edema, dyspnea, hyperventilation, edema of pharynx.
Skin and Appendages: rash4, pruritus4, alopecia, urticaria, eczema, dermatitis, bullous eruption, erythema multiforme major, angioedema, Stevens-Johnson syndrome, excess perspiration, exfoliative dermatitis.
Special Senses: tinnitus4, blurred vision, taste disorder, reversible and irreversible hearing loss, scotoma, vitreous floaters, night blindness, amblyopia.
Urogenital: nephrotic syndrome, proteinuria, oliguria, interstitial nephritis, papillary necrosis, acute renal failure, urinary frequency, nocturia, hematuria, impotence, vaginal bleeding.
- 4 Incidence greater than 1% marked with asterisk.
-
OVERDOSAGE
Due to the low systemic absorption of topically-applied diclofenac sodium gel, overdosage is unlikely. There have been no reports of ingestion of diclofenac sodium gel. In the event of oral ingestion, resulting in significant systemic side effects, it is recommended that the stomach be emptied by vomiting or lavage. Forced diuresis may theoretically be beneficial because the drug is excreted in the urine. The effect of dialysis or hemoperfusion in the elimination of diclofenac (99% protein-bound) remains unproven. In addition to supportive measures, the use of oral activated charcoal may help to reduce the absorption of diclofenac. Supportive and symptomatic treatment should be given for complications such as renal failure, convulsions, gastrointestinal irritation and respiratory depression.
-
DOSAGE AND ADMINISTRATION
Diclofenac sodium gel is applied to lesion areas twice daily. It is to be smoothed onto the affected skin gently. The amount needed depends upon the size of the lesion site. Assure that enough diclofenac sodium gel is applied to adequately cover each lesion. Normally 0.5 g of gel is used on each 5 cm × 5 cm lesion site. The recommended duration of therapy is from 60 days to 90 days. Complete healing of the lesion(s) or optimal therapeutic effect may not be evident for up to 30 days following cessation of therapy. Lesions that do not respond to therapy should be carefully re-evaluated and management reconsidered.
-
HOW SUPPLIED
Diclofenac Sodium Gel, 3% is available in 100 g (NDC: 45861-063-01) tubes. Each gram of gel contains 30 mg of diclofenac sodium.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 100 g Tube Carton
NDC: 45861-063-01
100 g
Diclofenac Sodium
Gel 3%FOR EXTERNAL USE ONLY.
NOT FOR OPHTHALMIC USE.Keep this and all medications out of the reach of children.
Rx only

-
INGREDIENTS AND APPEARANCE
DICLOFENAC SODIUM
diclofenac sodium gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45861-063 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength diclofenac sodium (UNII: QTG126297Q) (diclofenac - UNII:144O8QL0L1) diclofenac sodium 30 mg in 1 g Inactive Ingredients Ingredient Name Strength benzyl alcohol (UNII: LKG8494WBH) hyaluronate sodium (UNII: YSE9PPT4TH) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45861-063-01 1 in 1 CARTON 04/28/2016 1 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206298 04/28/2016 Labeler - Pharmaceutica North America, Inc (962739699)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.