EYLEA HD- aflibercept injection, solution
EYLEA HD by
Drug Labeling and Warnings
EYLEA HD by is a Prescription medication manufactured, distributed, or labeled by Regeneron Pharmaceuticals, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EYLEA HD safely and effectively. See full prescribing information for EYLEA HD.
EYLEA HD® (aflibercept) injection, for intravitreal use
Initial U.S. Approval: 2011RECENT MAJOR CHANGES
INDICATIONS AND USAGE
EYLEA HD is a vascular endothelial growth factor (VEGF) inhibitor indicated for the treatment of patients with:
DOSAGE AND ADMINISTRATION
-
Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
- The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 to 16 weeks, +/- 1 week. (2.2)
- Some patients did not maintain a response with extended dosing intervals after successful response to the three initial monthly doses. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days). (2.2)
-
Diabetic Macular Edema (DME)
- The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 to 16 weeks, +/- 1 week. (2.3)
- Some patients did not maintain a response with extended dosing intervals after successful response to the three initial monthly doses. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days). (2.3)
-
Diabetic Retinopathy (DR)
- The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 to 12 weeks, +/- 1 week. (2.4)
- Some patients did not maintain a response with extended dosing intervals after successful response to the three initial monthly doses. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days). (2.4)
-
Macular Edema Following Retinal Vein Occlusion (RVO)
- The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three to five doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 weeks, +/- 1 week. (2.5)
- Some patients did not maintain a response with extended dosing intervals after successful response to the first three to five initial monthly doses. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days). (2.5)
DOSAGE FORMS AND STRENGTHS
- Injection: 8 mg (0.07 mL of 114.3 mg/mL solution) in a single-dose vial (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Endophthalmitis, retinal detachments, and retinal vasculitis with or without occlusion may occur following intravitreal injections. Patients should be instructed to report any symptoms suggestive of endophthalmitis, retinal detachment, or retinal vasculitis without delay and should be managed appropriately. (5.1)
- Increases in intraocular pressure have been seen within 60 minutes of an intravitreal injection. (5.2)
- There is a potential risk of arterial thromboembolic events following intravitreal use of VEGF inhibitors. (5.3)
ADVERSE REACTIONS
The most common adverse reactions (≥3%) reported in patients treated with EYLEA HD were cataract, conjunctival hemorrhage, corneal epithelium defect, intraocular pressure increased, ocular discomfort/eye pain/eye irritation, retinal hemorrhage, vision blurred, vitreous detachment, and vitreous floaters. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Regeneron at 1-855-395-3248 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2025
-
Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
1.2 Diabetic Macular Edema (DME)
1.3 Diabetic Retinopathy (DR)
1.4 Macular Edema Following Retinal Vein Occlusion (RVO)
2 DOSAGE AND ADMINISTRATION
2.1 Important Injection Instructions
2.2 Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
2.3 Diabetic Macular Edema (DME)
2.4 Diabetic Retinopathy (DR)
2.5 Macular Edema Following Retinal Vein Occlusion (RVO)
2.6 Preparation for Administration
2.7 Injection Procedure
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Ocular or Periocular Infections
4.2 Active Intraocular Inflammation
4.3 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Endophthalmitis, Retinal Detachments, and Retinal Vasculitis with or without Occlusion
5.2 Increase in Intraocular Pressure
5.3 Thromboembolic Events
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
14.2 Diabetic Macular Edema (DME)
14.3 Diabetic Retinopathy (DR)
14.4 Macular Edema Following Retinal Vein Occlusion (RVO)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Injection Instructions
For ophthalmic intravitreal injection. EYLEA HD must only be administered by a qualified physician.
A 5-micron sterile filter needle (18-gauge × 1½-inch), a 1-mL Luer lock syringe and a 30-gauge × ½-inch sterile injection needle are needed.
EYLEA HD is available packaged as follows:
- Vial Only
- Vial Kit with Injection Components (filter needle, syringe, injection needle)
2.2 Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 to 16 weeks, +/- 1 week.
Some patients did not maintain a response with extended dosing intervals after successful response to the three initial monthly doses [see Clinical Studies (14.1)]. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days).
2.3 Diabetic Macular Edema (DME)
The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 to 16 weeks, +/- 1 week.
Some patients did not maintain a response with extended dosing intervals after successful response to the three initial monthly doses [see Clinical Studies (14.2)]. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days).
2.4 Diabetic Retinopathy (DR)
The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 to 12 weeks, +/- 1 week.
Some patients did not maintain a response with extended dosing intervals after successful response to the three initial monthly doses [see Clinical Studies (14.3)]. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days).
2.5 Macular Edema Following Retinal Vein Occlusion (RVO)
The recommended dose for EYLEA HD is 8 mg (0.07 mL of 114.3 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days +/- 7 days) for the first three to five doses, followed by 8 mg (0.07 mL of 114.3 mg/mL solution) via intravitreal injection once every 8 weeks, +/- 1 week.
Some patients did not maintain a response with extended dosing intervals after successful response to the first three to five initial monthly doses [see Clinical Studies (14.4)]. These patients may benefit from resuming every 4-week dosing (approximately every 28 days +/- 7 days).
2.6 Preparation for Administration
The EYLEA HD glass vial is for one-time use in one eye only. Discard unused portion. EYLEA HD does not contain an anti-microbial preservative. Extraction of multiple doses from a single vial may increase the risk of contamination and subsequent infection.
Do not use if the package or its components are expired, damaged, or have been tampered with.
Check the label on the vial to make sure you have the correct aflibercept strength.
Prepare for intravitreal injection with the following medical devices for single use.
- a 5-micron sterile filter needle (18-gauge × 1½-inch)
- a 1-mL sterile Luer lock syringe (with marking to measure 0.07 mL)
- a sterile injection needle (30-gauge × ½-inch)
- 1. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the vial if particulates, cloudiness, or discoloration are visible.
- 2. Remove the protective plastic cap from the vial (see Figure 1).
- 3. Clean the top of the vial with an alcohol wipe (see Figure 2).
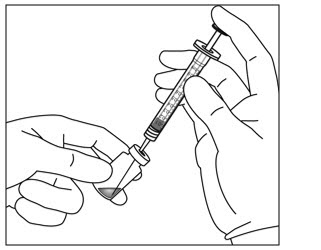
- 4. Use aseptic technique to carry out steps 4 – 11. Remove the 18-gauge × 1½-inch, 5-micron, filter needle and the 1-mL syringe from their packaging. Attach the filter needle to the syringe by twisting it onto the Luer lock syringe tip (see Figure 3).
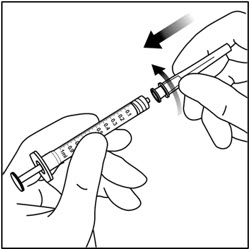
- 5. Push the filter needle into the center of the vial stopper until the needle is completely inserted into the vial and the tip touches the bottom or bottom edge of the vial.
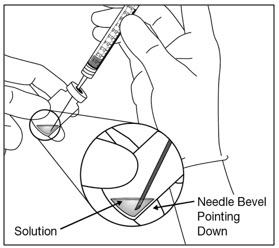
- 6. Withdraw all of the EYLEA HD vial contents into the syringe, keeping the vial in an upright position, slightly inclined to ease complete withdrawal. To deter the introduction of air, ensure the bevel of the filter needle is submerged into the liquid. Continue to tilt the vial during withdrawal keeping the bevel of the filter needle submerged in the liquid (see Figure 4a and Figure 4b).
- 7. Ensure that the plunger rod is drawn sufficiently back when emptying the vial in order to completely empty the filter needle.
- 8. Remove the filter needle from the syringe and properly dispose of the filter needle. Note: Filter needle is not to be used for intravitreal injection.
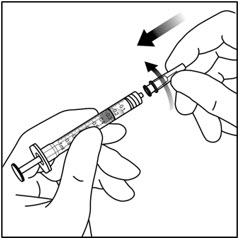
- 9. Remove the 30-gauge × ½-inch injection needle from its packaging and attach the injection needle to the syringe by firmly twisting the injection needle onto the Luer lock syringe tip (see Figure 5).
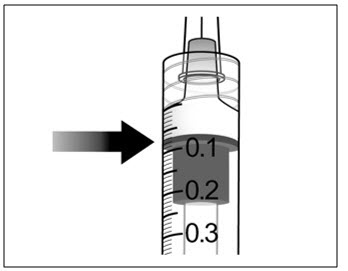
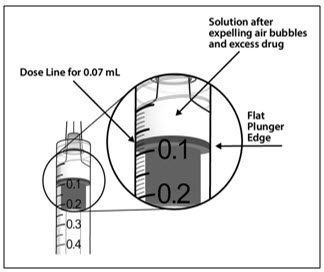
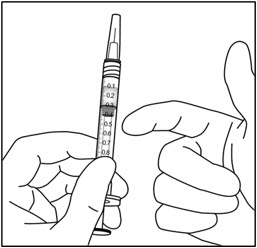
- 10. Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure 6).
2.7 Injection Procedure
The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include surgical hand disinfection and the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a topical broad–spectrum microbicide should be given prior to the injection.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure. Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, a sterile paracentesis needle should be available.
Following intravitreal injection, patients and/or caregivers should be instructed to report any signs and/or symptoms suggestive of endophthalmitis or retinal detachment (e.g., eye pain, redness of the eye, photophobia, blurring of vision) without delay [see Patient Counseling Information (17)].
Each vial should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new vial should be used and the sterile field (including a new syringe, gloves, drapes, eyelid speculum, filter and injection needles) should be changed before EYLEA HD is administered to the other eye.
After injection, discard any unused product or waste material in accordance with local regulations.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Endophthalmitis, Retinal Detachments, and Retinal Vasculitis with or without Occlusion
Intravitreal injections including those with aflibercept have been associated with endophthalmitis and retinal detachments [see Adverse Reactions (6.1)] and, more rarely, retinal vasculitis with or without occlusion [see Adverse Reactions (6.2)]. Proper aseptic injection technique must always be used when administering EYLEA HD. Patients and/or caregivers should be instructed to report any signs and/or symptoms suggestive of endophthalmitis, retinal detachment, or retinal vasculitis without delay and should be managed appropriately [see Dosage and Administration (2.7) and Patient Counseling Information (17)].
5.2 Increase in Intraocular Pressure
Acute increases in intraocular pressure have been seen within 60 minutes of intravitreal injection, including with EYLEA HD [see Adverse Reactions (6.1)]. Sustained increases in intraocular pressure have also been reported after repeated intravitreal dosing with vascular endothelial growth factor (VEGF) inhibitors. Intraocular pressure and the perfusion of the optic nerve head should be monitored and managed appropriately [see Dosage and Administration (2.7)].
5.3 Thromboembolic Events
There is a potential risk of arterial thromboembolic events (ATEs) following intravitreal use of VEGF inhibitors, including EYLEA HD. ATEs are defined as nonfatal stroke, nonfatal myocardial infarction, or vascular death (including deaths of unknown cause). The incidence of reported thromboembolic events in the wet AMD study (PULSAR) from baseline through week 48 was 0.4% (3 out of 673) in the combined group of patients treated with EYLEA HD compared with 1.5% (5 out of 336) in patients treated with EYLEA 2 mg. The incidence of reported thromboembolic events in the DME study (PHOTON) from baseline to week 48 was 3.1% (15 out of 491) in the combined group of patients treated with EYLEA HD compared with 3.6% (6 out of 167) in patients treated with EYLEA 2 mg. The incidence of reported thromboembolic events in the RVO study (QUASAR) from baseline to week 36 was 0.5% (3 out of 591) in the combined group of patients treated with EYLEA HD compared with 1.7% (5 out of 301) in patients treated with EYLEA 2 mg.
-
6 ADVERSE REACTIONS
The following potentially serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity [see Contraindications (4.3)]
- Endophthalmitis, Retinal Detachments, and Retinal Vasculitis with or without Occlusion [see Warnings and Precautions (5.1)]
- Increase in intraocular pressure [see Warnings and Precautions (5.2)]
- Thromboembolic events [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in other clinical trials of the same or another drug and may not reflect the rates observed in practice.
A total of 1755 patients were treated with EYLEA HD and 804 patients were treated with EYLEA 2 mg in three clinical studies. The most common adverse reactions reported in ≥3% of patients treated with EYLEA HD were cataract, conjunctival hemorrhage, corneal epithelium defect, intraocular pressure increased, ocular discomfort/eye pain/eye irritation, retinal hemorrhage, vision blurred, vitreous detachment and vitreous floaters.
Neovascular (Wet) Age-Related Macular Degeneration (AMD) and Diabetic Macular Edema (DME)
The data described below reflect exposure to EYLEA HD administered every 12 weeks (HDq12), EYLEA HD administered every 16 weeks (HDq16), or EYLEA 2 mg administered every 8 weeks (2q8) in controlled clinical studies (PULSAR and PHOTON), each for 48 weeks [see Clinical Studies (14.1, 14.2)].
Table 1: Adverse Reactions (≥1%) in at least one group in the PULSAR or PHOTON studies Adverse Reactions PULSAR PHOTON EYLEA
HDq12EYLEA
HDq16EYLEA
2q8EYLEA
HDq12EYLEA
HDq16EYLEA
2q8n=335 n=338 n=336 n=328 n=163 n=167 - * Represents grouping of related terms
Cataract* 4% 4% 4% 3% 6% 3% Conjunctival hemorrhage* 3% 2% 1% 4% 4% 4% Intraocular pressure increased* 4% 4% 2% 3% 1% 4% Ocular discomfort/eye pain/eye irritation* 3% 3% 2% 4% 2% 4% Vision blurred* 4% 6% 7% 3% 3% 4% Vitreous floaters* 1% 4% 3% 5% 2% 3% Vitreous detachment* 2% 3% 2% 4% 2% 1% Corneal epithelium defect* 2% 2% 3% 3% 6% 1% Retinal hemorrhage 3% 3% 4% 0 4% 1% Intraocular inflammation* 1% 1% 1% 1% 0 1% Retinal pigment epithelial tear/epitheliopathy* 2% 1% 2% <1% 0 0 Vitreous hemorrhage <1% 1% 1% 2% 1% 1% Retinal Detachment* 1% <1% 0 <1% 1% 0 Foreign body sensation in eyes* 1% 1% 2% <1% 0 0 Retinal pigment epithelial detachment* 1% 1% 2% 0 0 0 Adverse drug reactions (ADRs) reported in <1% of participants treated with EYLEA HD were ocular hyperemia (includes adverse events of conjunctival hyperemia, conjunctival irritation, ocular hyperemia), lacrimation increased, eyelid edema, hypersensitivity (includes adverse events of rash, urticaria, pruritus), retinal tear and injection site hemorrhage.
Macular Edema Following Retinal Vein Occlusion (RVO)
The data described below reflects 36 weeks exposure to EYLEA HD administered every 8 weeks (HDq8) after 3 or 5 initial monthly doses (HDq4), or EYLEA 2 mg administered every 4 weeks (2q4) in a controlled clinical study (QUASAR). [see Clinical Studies (14.4)].
Table 2: Most Common Adverse Reactions (≥1%) in at least one group in the QUASAR study Adverse Reactions EYLEA HDq8 following 3 initial doses (HDq4)
(N=293)EYLEA HDq8 following 5 initial doses (HDq4)
(N=298)EYLEA 2q4
(N=301)- * Represents grouping of related terms
- † Represents reported non-ocular adverse events of hypersensitivity, rash, urticaria and pruritus
Intraocular pressure increased* 7% 6% 3% Vision blurred* 5% 3% 2% Conjunctival hemorrhage* 3% 2% 2% Ocular discomfort/eye pain/eye irritation* 3% 3% 1% Vitreous detachment* 3% 3% 1% Cataract* 2% 4% 3% Corneal epithelium defect* 2% 2% 2% Dry eye* 2% 2% 2% Vitreous floaters* 1% 1% 1% Intraocular inflammation* 1% <1% 1% Vitreous hemorrhage 1% 1% 0 Hypersensitivity† 1% 1% 1% Adverse reactions reported in <1% of the patients treated with EYLEA HD in the RVO study were foreign body sensation in eyes (includes foreign body sensation in eyes and sensation of foreign body), ocular hyperaemia (includes conjunctival hyperemia, conjunctival irritation, ocular hyperemia), retinal hemorrhage, retinal pigment epithelial detachment (includes detachment of retinal pigment epithelium), retinal pigment epithelial tear/epitheliopathy (includes retinal pigment epitheliopathy), and retinal tear.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of aflibercept. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye disorders:
- Retinal vasculitis and occlusive retinal vasculitis related to intravitreal injection with aflibercept (reported at a rate of 0.6 and 0.2 per 1 million injections, respectively, based on postmarketing experience from November 2011 until November 2023).
- Scleritis.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Adequate and well-controlled studies with EYLEA HD have not been conducted in pregnant women. Aflibercept produced adverse embryofetal effects in rabbits, including external, visceral, and skeletal malformations. A fetal No Observed Adverse Effect Level (NOAEL) was not identified. At the lowest dose shown to produce adverse embryofetal effects, systemic exposure (based on AUC for free aflibercept) was approximately 0.9 -fold of the population pharmacokinetic estimated exposure in humans after an intravitreal dose of 8 mg (see Data).
Animal reproduction studies are not always predictive of human response, and it is not known whether EYLEA HD can cause fetal harm when administered to a pregnant woman. Based on the anti-VEGF mechanism of action for aflibercept [see Clinical Pharmacology (12.1)], treatment with EYLEA HD may pose a risk to human embryofetal development. EYLEA HD should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In two embryofetal development studies, aflibercept produced adverse embryofetal effects when administered every three days during organogenesis to pregnant rabbits at intravenous doses ≥3 mg per kg, or every six days during organogenesis at subcutaneous doses ≥0.1 mg per kg.
Adverse embryofetal effects included increased incidences of postimplantation loss and fetal malformations, including anasarca, umbilical hernia, diaphragmatic hernia, gastroschisis, cleft palate, ectrodactyly, intestinal atresia, spina bifida, encephalomeningocele, heart and major vessel defects, and skeletal malformations (fused vertebrae, sternebrae, and ribs; supernumerary vertebral arches and ribs; and incomplete ossification). The maternal No Observed Adverse Effect Level (NOAEL) in these studies was 3 mg per kg. Aflibercept produced fetal malformations at all doses assessed in rabbits and the fetal NOAEL was not identified. At the lowest dose shown to produce adverse embryofetal effects in rabbits (0.1 mg per kg), systemic exposure (AUC) of free aflibercept was approximately 0.9-fold of the population pharmacokinetic estimated systemic exposure (AUC) in humans after an intravitreal dose of 8 mg.
8.2 Lactation
Risk Summary
There is no information regarding the presence of aflibercept in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production/excretion. Because many drugs are excreted in human milk, and because the potential for absorption and harm to infant growth and development exists, EYLEA HD is not recommended during breastfeeding.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EYLEA HD and any potential adverse effects on the breastfed child from EYLEA HD.
8.3 Females and Males of Reproductive Potential
Contraception
Females of reproductive potential are advised to use effective contraception prior to the initial dose, during treatment, and for at least 4 months after the last intravitreal injection of EYLEA HD.
Infertility
There are no data regarding the effects of EYLEA HD on human fertility. Aflibercept adversely affected female and male reproductive systems in cynomolgus monkeys when administered by intravenous injection at a dose 91 times higher (based on AUC of free aflibercept) than the corresponding systemic level estimated based on population pharmacokinetic analysis in humans following an intravitreal dose of 8 mg. A No Observed Adverse Effect Level (NOAEL) was not identified. These findings were reversible within 20 weeks after cessation of treatment [see Nonclinical Toxicology (13.1)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Aflibercept is a recombinant fusion protein consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG1 formulated as an iso-osmotic solution for intravitreal administration. Aflibercept is a dimeric glycoprotein and contains glycosylation, constituting an additional 15% of the total molecular mass, resulting in a total molecular weight of 115 kDa. Aflibercept is produced in recombinant Chinese hamster ovary (CHO) cells.
EYLEA HD (aflibercept) injection is a sterile, clear to slightly opalescent, and colorless to pale yellow solution. EYLEA HD is supplied as a sterile, aqueous solution for intravitreal injection in a single-dose glass vial designed to deliver 0.07 mL (70 microliters) of solution containing 8 mg of aflibercept in a buffer containing arginine hydrochloride (0.737 mg), histidine (0.04 mg), L-histidine hydrochloride monohydrate (0.093 mg), polysorbate 20 (0.021 mg), sucrose (3.5 mg) and water for injection with a pH of 5.8. EYLEA HD does not contain an anti-microbial preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PlGF) are members of the VEGF family of angiogenic factors that can act as mitogenic, chemotactic, and vascular permeability factors for endothelial cells. VEGF acts via two receptor tyrosine kinases, VEGFR-1 and VEGFR-2, present on the surface of endothelial cells. PlGF binds only to VEGFR-1, which is also present on the surface of leucocytes. Activation of these receptors by VEGF-A can result in neovascularization and vascular permeability.
Aflibercept acts as a soluble decoy receptor that binds VEGF-A and PlGF, and thereby can inhibit the binding and activation of these cognate VEGF receptors.
12.2 Pharmacodynamics
Increased retinal thickness, assessed by optical coherence tomography (OCT), is associated with nAMD, DME, and RVO. Reductions in central subfield thickness (CST) were observed across all treatment arms throughout the three Phase 3 studies in nAMD, DME, and RVO.
12.3 Pharmacokinetics
EYLEA HD is administered intravitreally to exert local effects in the eye. In patients with wet AMD, or DME, following intravitreal administration of EYLEA HD, a fraction of the administered dose is expected to bind with endogenous VEGF in the eye to form an inactive aflibercept: VEGF complex. Once absorbed into the systemic circulation, aflibercept presents in the plasma as free aflibercept (unbound to VEGF) and a more predominant stable inactive form with circulating endogenous VEGF (i.e., aflibercept: VEGF complex).
Absorption/Distribution
As no relevant differences in pharmacokinetics between the nAMD, DME, and RVO populations were observed based on a population pharmacokinetic analysis of the data, population pharmacokinetic estimated parameters are presented for the combined populations. Following unilateral intravitreal administration of 8 mg aflibercept, the mean (SD) Cmax of free aflibercept in plasma was 0.32 (0.27) mg/L, and the median time to maximal concentration in plasma was 2.9 days. The accumulation of free aflibercept in plasma following three initial monthly intravitreal doses was minimal (mean accumulation ratio 1.2); subsequently, no further accumulation was observed.
The volume of distribution of free aflibercept following intravenous (I.V.) administration of aflibercept is approximately 7 L.
Metabolism/Elimination
Aflibercept is a therapeutic protein and no drug metabolism studies have been conducted. Aflibercept is expected to undergo elimination through both target-mediated disposition via binding to free endogenous VEGF and metabolism via proteolysis. The median time to reach non-quantifiable concentrations of free aflibercept in plasma for 8 mg administered intravitreally was 3.5 weeks.
Specific Populations
Renal and Hepatic Impairment
Population pharmacokinetic analysis revealed that systemic exposures to aflibercept in patients with mild to severe renal impairment (eGFR 15 to < 90 mL/min, estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for eGFR were similar to those with normal renal function. Mild hepatic impairment had no influence on systemic exposures to aflibercept compared to patients with normal hepatic function. No data for patients with moderate and severe hepatic impairment are available. No dose adjustment based on renal or hepatic impairment status is needed.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies for other products.
During the 48-week treatment with aflibercept administrated IVT, the incidence of anti-aflibercept antibody formation in the 8 mg treatment groups was 2.7% (25/937 participants with nAMD [PULSAR] or DME [PHOTON]).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted on the mutagenic or carcinogenic potential of aflibercept. Effects on male and female fertility were assessed as part of a 6-month study in monkeys with intravenous administration of aflibercept at weekly doses ranging from 3 to 30 mg per kg. Absent or irregular menses associated with alterations in female reproductive hormone levels and changes in sperm morphology and motility were observed at all dose levels. In addition, females showed decreased ovarian and uterine weight accompanied by compromised luteal development and reduction of maturing follicles. These changes correlated with uterine and vaginal atrophy. All changes were reversible within 20 weeks after cessation of treatment. A No Observed Adverse Effect Level (NOAEL) was not identified. Intravenous administration of the lowest dose of aflibercept assessed in monkeys (3 mg per kg) resulted in systemic exposure (AUC) for free aflibercept that was 91 times higher than the population pharmacokinetic estimated systemic exposure in humans after an intravitreal dose of 8 mg.
13.2 Animal Toxicology and/or Pharmacology
Erosions and ulcerations of the respiratory epithelium in nasal turbinates in monkeys treated with aflibercept intravitreally were observed at intravitreal doses of 2, 4 or 7 mg per eye. At the NOAEL of 0.5 mg per eye in monkeys, the systemic exposure (AUC) for free aflibercept was approximately 3 times higher than the population pharmacokinetic estimated exposure observed in humans after an intravitreal dose of 8 mg. Similar effects were not seen in clinical studies [see Clinical Studies (14)].
-
14 CLINICAL STUDIES
14.1 Neovascular (Wet) Age-Related Macular Degeneration (nAMD)
The safety and efficacy of EYLEA HD were assessed in a randomized, multi-center, double-masked, active-controlled study (PULSAR) in treatment-naïve patients with nAMD. A total of 1009 patients were treated and analyzed for efficacy (673 with EYLEA HD). Patients were randomly assigned in a 1:1:1 ratio to 1 of 3 treatment groups: 1) EYLEA HD administered every 12 weeks following 3 initial monthly doses (HDq12); 2) EYLEA HD administered every 16 weeks following 3 initial monthly doses (HDq16); 3) EYLEA 2 mg administered every 8 weeks (2q8) following 3 initial monthly doses. In the EYLEA HD groups, patients could be treated as frequently as every 8 weeks based on protocol-defined visual and anatomic criteria, starting at week 16. Patients ranged from 50 to 96 years of age with a mean of 74.5 years. At baseline, mean visual acuity was approximately 60 letters (range: 24 to 78 letters).
The primary efficacy endpoint was the change from baseline in BCVA at week 48 as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) letter score.
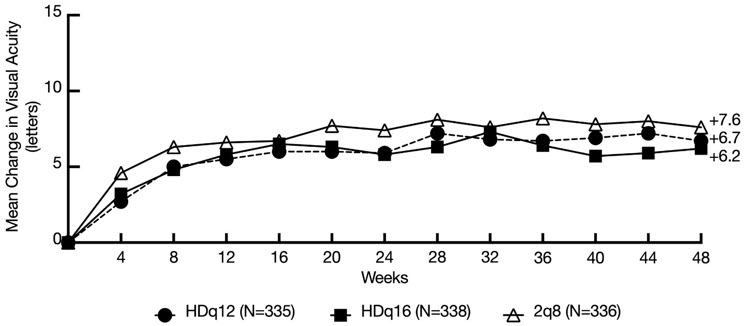
Both HDq12 and HDq16 treatments were shown to be non-inferior and clinically equivalent to 2q8 treatment with respect to the change in BCVA score at week 48 using the pre-specified non-inferiority margin of 4 letters. 6.2% of total EYLEA HD treated patients who met protocol-defined criteria to be treated every 8 weeks did not maintain a response with every 8 weeks dosing after successful response to the 3 initial monthly doses. In patients completing week 48, the mean number of injections administered were 5.2 in the HDq16 group (n=312), 6.1 in the HDq12 group (n=316) and 6.9 in the EYLEA q8 group (n=309). Detailed results from the analysis of the PULSAR study are shown in Table 3 and Figure 8 below.
Efficacy results in all subgroups (e.g., age, gender, geographic region, ethnicity, race, baseline BCVA and lesion type) were consistent with those in the overall population.
Table 3: Efficacy Outcomes (Full Analysis Set) in PULSAR Study Efficacy Outcomes EYLEA HDq12 EYLEA HDq16 EYLEA 2q8 BCVA = Best Corrected Visual Acuity; ETDRS = Early Treatment Diabetic Retinopathy Study; SD = Standard Deviation; LS = Least Square; SE = Standard Error; CI = Confidence Interval; MMRM = Mixed Model for Repeated Measurements - * Full Analysis Set (FAS) includes all randomized patients who received at least 1 dose of study medication
- † Observed values at week 48: n=299 for HDq12; n=289 for HDq16; n=285 for 2q8
- ‡ Estimate based on the MMRM model, was computed for the differences of HDq12 minus 2q8 and HDq16 minus 2q8, respectively, with two-sided 95% CIs
Full Analysis Set* N=335 N=338 N=336 Mean change in BCVA as measured by ETDRS letter score from baseline (SD) at week 48† 6.7
(12.6)6.2
(11.7)7.6
(12.2)LS mean (SE) change from baseline ‡ 6.1
(0.8)5.9
(0.7)7.0
(0.7)Difference in LS mean
(95% CI)‡-1.0
(-2.9, 0.9)-1.1
(-3.0, 0.7)Figure 8: Mean Change from Baseline in BCVA as measured by ETDRS Letter Score by Visits through Week 48 (Observed Cases)
14.2 Diabetic Macular Edema (DME)
The safety and efficacy of EYLEA HD was assessed in a randomized, multi-center, double-masked, active-controlled study (PHOTON) in patients with DME involving the center of the macula. A total of 658 patients were treated and analyzed for efficacy (491 with EYLEA HD). Patients were randomly assigned in a 2:1:1 ratio to 1 of 3 treatment groups: 1) EYLEA HD administered every 12 weeks following 3 initial monthly doses (HDq12); 2) EYLEA HD administered every 16 weeks following 3 initial monthly doses (HDq16); 3) EYLEA 2 mg administered every 8 weeks (2q8) following 5 initial monthly doses. In the EYLEA HD groups, patients could be treated as frequently as every 8 weeks based on protocol-defined visual and anatomic criteria, starting at week 16. Patient ages ranged from 24 to 90 years with a mean of 62.3 years. A total of 44% of patients were previously treated for DME. At baseline, the overall mean visual acuity was 63 letters (range: 24 to 79 letters).
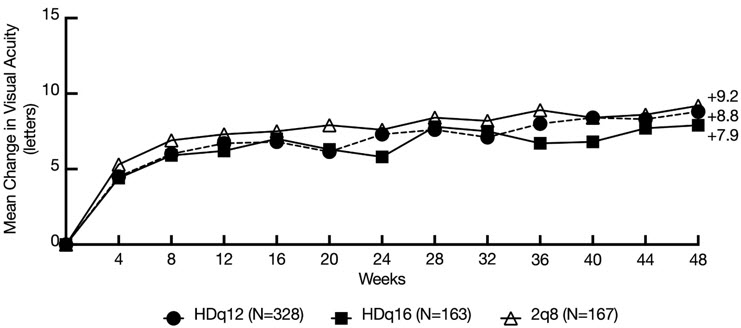
The primary efficacy endpoint was the change from baseline in BCVA at week 48 as measured by the ETDRS letter score. Both HDq12 and HDq16 treatments were shown to be non-inferior and clinically equivalent to 2q8 treatment with respect to the change in BCVA score at week 48 using the pre-specified non-inferiority margin of 4 letters. 1.5% of total EYLEA HD treated patients who met protocol-defined criteria to be treated every 8 weeks did not maintain a response with every 8 weeks dosing after successful response to the 3 initial monthly doses. In patients completing week 48, the mean number of injections administered were 5.0 in the HDq16 group (n=155), 6.0 in the HDq12 group (n=300) and 7.9 in the EYLEA q8 group (n=157). Detailed results from the analysis of the PHOTON study are shown in Table 4 and Figure 9 below.
Table 4: Efficacy Outcomes (Full Analysis Set) in PHOTON Study Efficacy Outcomes EYLEA HDq12 EYLEA HDq16 EYLEA 2q8 BCVA = Best Corrected Visual Acuity; ETDRS = Early Treatment Diabetic Retinopathy Study; SD = Standard Deviation; LS = Least Square; SE = Standard Error; CI = Confidence Interval; MMRM = Mixed Model for Repeated Measurements. - * FAS includes all randomized patients who received at least 1 dose of study medication
- † Observed values at week 48: n=277 for HDq12; n=149 for HDq16; n=150 for 2q8
- ‡ Estimate based on the MMRM model, was computed for the differences of HDq12 minus 2q8 and HDq16 minus 2q8, respectively with two-sided 95% CIs
Full Analysis Set* N=328 N=163 N=167 Mean change in BCVA as measured by ETDRS letter score from baseline (SD) at week 48† 8.8 (9.0) 7.9 (8.4) 9.2 (9.0) LS mean (SE) change from baseline‡ 8.1 (0.6) 7.2 (0.7) 8.7 (0.7) Difference in LS mean (95% CI)‡ -0.6
(-2.3, 1.1)-1.4
(-3.3, 0.4)Efficacy results in all subgroups (e.g., age, gender, geographic region, ethnicity, race, baseline, BCVA, baseline CRT and prior DME treatment) were consistent with those in the overall population.
Figure 9: Mean Change from Baseline in BCVA as measured by ETDRS Letter Score by Visits through Week 48 (Observed Cases)
14.3 Diabetic Retinopathy (DR)
Efficacy and safety data of EYLEA HD in diabetic retinopathy (DR) are derived from the PHOTON study.
In the PHOTON study, a key efficacy outcome was the change in the Early Treatment Diabetic Retinopathy Study (ETDRS) Diabetic Retinopathy Severity Scale (ETDRS-DRSS). Each EYLEA HD group was compared to the 2q8 group using a NI margin of 10%.
The ETDRS-DRSS score was assessed at baseline and approximately every 3 months thereafter for the duration of the study [see Clinical Studies (14.2)]. Baseline ETDRS-DRSS scores were generally balanced across treatment groups. 1.5% of total EYLEA HD treated patients who met protocol-defined criteria to be treated every 8 weeks did not maintain a response with every 8 weeks dosing after successful response to the 3 initial monthly doses.
Results from the analysis of ETDRS-DRSS scores at week 48 in the PHOTON study are shown in Table 5 below:
Table 5: Proportion of Patients Who Achieved a ≥2-Step Improvement from Baseline in the ETDRS-DRSS Score at Week 48 (Full Analysis Set) in PHOTON Efficacy Outcomes EYLEA HDq12 EYLEA HDq16 EYLEA 2q8 Missing or non-gradable post-baseline ETDRS-DRSS values were imputed using the last gradable ETDRS-DRSS values. Patients were considered as non-responders if all post-baseline measurements were missing or non-gradable. Missing or ungradable baseline was not included in the denominator. - * FAS includes all randomized patients who received at least 1 dose of study medication
- † Last observation carried forward
- ‡ Difference with confidence interval (CI) was calculated using Mantel-Haenszel weighting scheme
Full Analysis Set* N=328 N=163 N=167 Proportion of patients with a ≥2-step improvement on ETDRS-DRSS from Baseline (%)† 29% 20% 27% Difference‡(%)
(95% CI)2%
(-6.6, 10.6)-8%
(-16.9, 1.8)The EYLEA HDq16 did not meet the non-inferiority criteria for the proportion of patients with a ≥2-step improvement on ETDRS-DRSS and is not considered clinically equivalent to EYLEA administered every 8 weeks.
Results of the subgroups (e.g., age, gender, race, ethnicity, baseline BCVA and prior DME treatment) on the proportion of patients who achieved a ≥2-step improvement on the ETDRS-DRSS from baseline to week 48 were, in general, consistent with those in the overall population.
14.4 Macular Edema Following Retinal Vein Occlusion (RVO)
The safety and efficacy of EYLEA HD was assessed in a randomized, multi-center, double-masked, active-controlled study (QUASAR) in patients with treatment naïve macular edema secondary to RVO. A total of 892 patients (425 with CRVO/HRVO and 467 with BRVO) were treated and analyzed for efficacy (591 with EYLEA HD). Patients were randomly assigned in a 1:1:1 ratio to 1 of 3 treatment groups: 1) EYLEA HD administered every 8 weeks, following 3 initial monthly doses (HDq8/3); 2) EYLEA HD administered every 8 weeks, following 5 initial monthly doses (HDq8/5); 3) EYLEA 2 mg administered every 4 weeks (2q4). Dosing intervals could be shortened or extended by 4-week increments based on protocol-defined visual and anatomic criteria. Intervals could be shortened beginning at week 16 for the HDq8/3 group, at week 24 for the HDq8/5 group and, if previously extended, at week 40 for the 2q4 group; intervals could be extended beginning at week 32 for the HDq8/3 and 2q4 groups and at week 40 for the HDq8/5 group. Patient ages ranged from 23 to 95 years with a mean of 65.9 years. At baseline, mean visual acuity was approximately 55 letters (range: 18 to 74 letters).
The primary efficacy endpoint was the change from baseline in BCVA at week 36 as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) letter score.
Both EYLEA HD groups were shown to be non-inferior and clinically equivalent to EYLEA with respect to the change in BCVA score at week 36 using the pre-specified non-inferiority margin of 4 letters. 9.1% of total EYLEA HD treated patients met protocol-defined criteria to be treated every 4 weeks. Detailed results from the analysis of the QUASAR study are shown in Table 6 and Figure 10 below.
Efficacy results in all subgroups (e.g., age, gender, geographic region, ethnicity, race, baseline BCVA and RVO subtype) were generally consistent with those in the overall population.
Table 6: Efficacy Outcomes (Full Analysis Set) in QUASAR Study Efficacy Outcomes EYLEA HDq8/3 EYLEA HDq8/5 EYLEA 2q4 Full Analysis Set* N=293 N=298 N=301 BCVA = Best Corrected Visual Acuity; ETDRS = Early Treatment Diabetic Retinopathy Study; SD = Standard Deviation; LS = Least Square; SE = Standard Error; CI = Confidence Interval; MMRM = Mixed Model for Repeated Measurements - * Full Analysis Set (FAS) includes all randomized patients who received at least 1 dose of study medication
- † Observed values at week 36: n=260 for HDq8/3; n=248 for HDq8/5; n=264 for 2q4
- ‡ Estimate based on the MMRM model, was computed for the differences of HDq8/3 minus 2q4 and HDq8/5 minus 2q4, respectively, with two-sided 95% CIs
Mean change in BCVA as measured by ETDRS letter score from baseline (SD) at week 36† 17.0
(11.8)19.1
(11.2)17.8
(13.1)LS mean (SE) change from baseline ‡ 17.0
(0.7)17.9
(0.6)17.1
(0.7)Difference in LS mean
(95% CI)‡-0.1
(-2.0, 1.9)0.8
(-1.1, 2.7)Figure 10: Mean Change from Baseline in BCVA as measured by ETDRS Letter Score by Visits through Week 36 (Observed Cases)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
EYLEA HD (aflibercept) is a clear to slightly opalescent, colorless to pale yellow solution supplied in the following presentations [see Dosage and Administration (2.6) and (2.7)]. Each vial is for single eye use only. Discard unused portion.
NDC NUMBER CARTON TYPE CARTON CONTENTS 61755-050-01 Vial Kit with Injection Components - one EYLEA HD 8 mg (0.07 mL of a 114.3 mg/mL solution), single-dose glass vial
- one 18-gauge × 1½-inch, 5-micron, filter needle for withdrawal of the vial contents
- one 30-gauge × ½-inch injection needle for intravitreal injection
- one 1-mL syringe for administration
- one Prescribing Information
61755-051-01 Vial Only - one EYLEA HD 8 mg (0.07 mL of a 114.3 mg/mL solution) single-dose glass vial
- one Prescribing Information
-
17 PATIENT COUNSELING INFORMATION
In the days following EYLEA HD administration, patients are at risk of developing endophthalmitis, retinal detachment, or retinal vasculitis with or without occlusion. If the eye becomes red, sensitive to light, painful, or develops a change in vision, advise patients and/or caregivers to seek immediate care from an ophthalmologist [see Warnings and Precautions (5.1)].
Patients may experience temporary visual disturbances after an intravitreal injection with EYLEA HD and the associated eye examinations [see Adverse Reactions (6.1)]. Advise patients not to drive or use machinery until visual function has recovered sufficiently.
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Regeneron Pharmaceuticals, Inc.
777 Old Saw Mill River Road
Tarrytown, NY 10591-6707
U.S. License Number 1760
For patent information: https://www.regeneron.com/downloads/us-patent-products.pdf
EYLEA HD is a registered trademark of Regeneron Pharmaceuticals, Inc.
©2025, Regeneron Pharmaceuticals, Inc.
All rights reserved.
Date: November 2025
-
PRINCIPAL DISPLAY PANEL - 0.07 mL Vial Carton - 050
NDC: 61755-050-01
Rx ONLY
EYLEA®HD
(aflibercept) Injection8 mg (0.07 mL of a 114.3 mg/mL solution)
For Intravitreal Injection.
Single-Dose Vial. Discard Unused Portion.Carton contents:
- one aflibercept 8 mg/0.07 mL single-dose glass vial
- one 18-gauge × 1½-inch, 5-micron, filter needle for withdrawal
of the vial contents - one 30-gauge × ½-inch injection needle for intravitreal injection
- one 1-mL syringe for administration
- one Prescribing Information
REGENERON
-
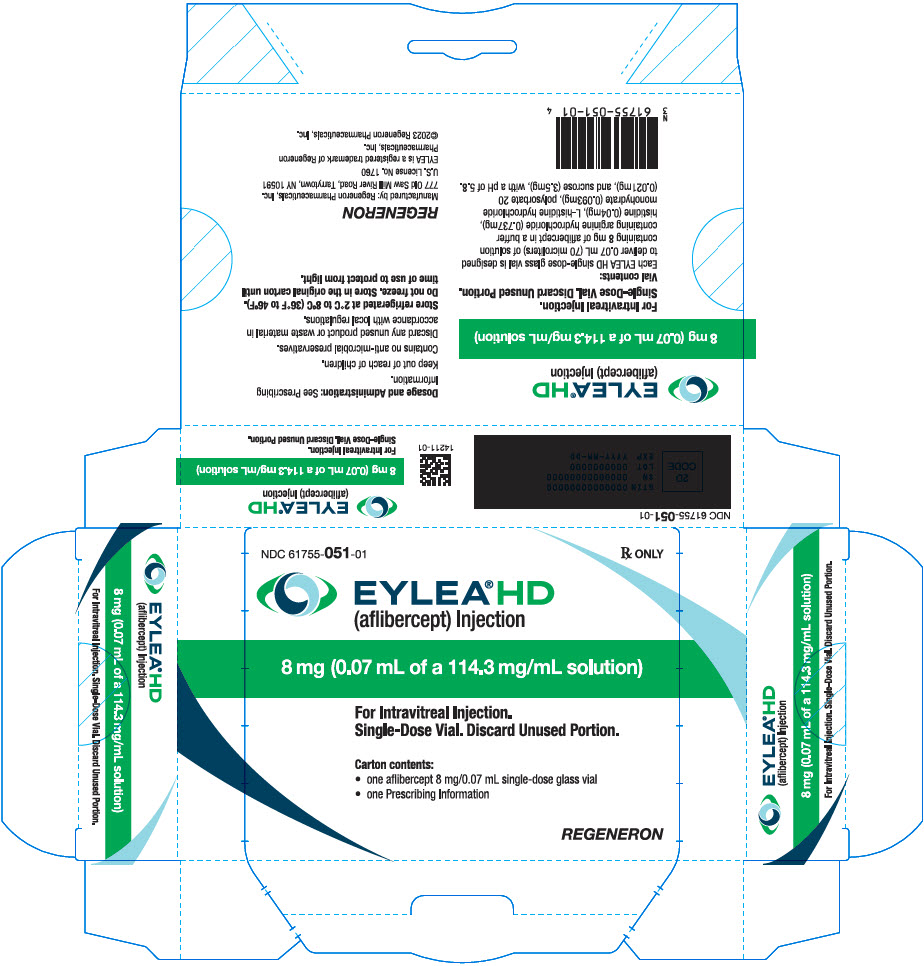
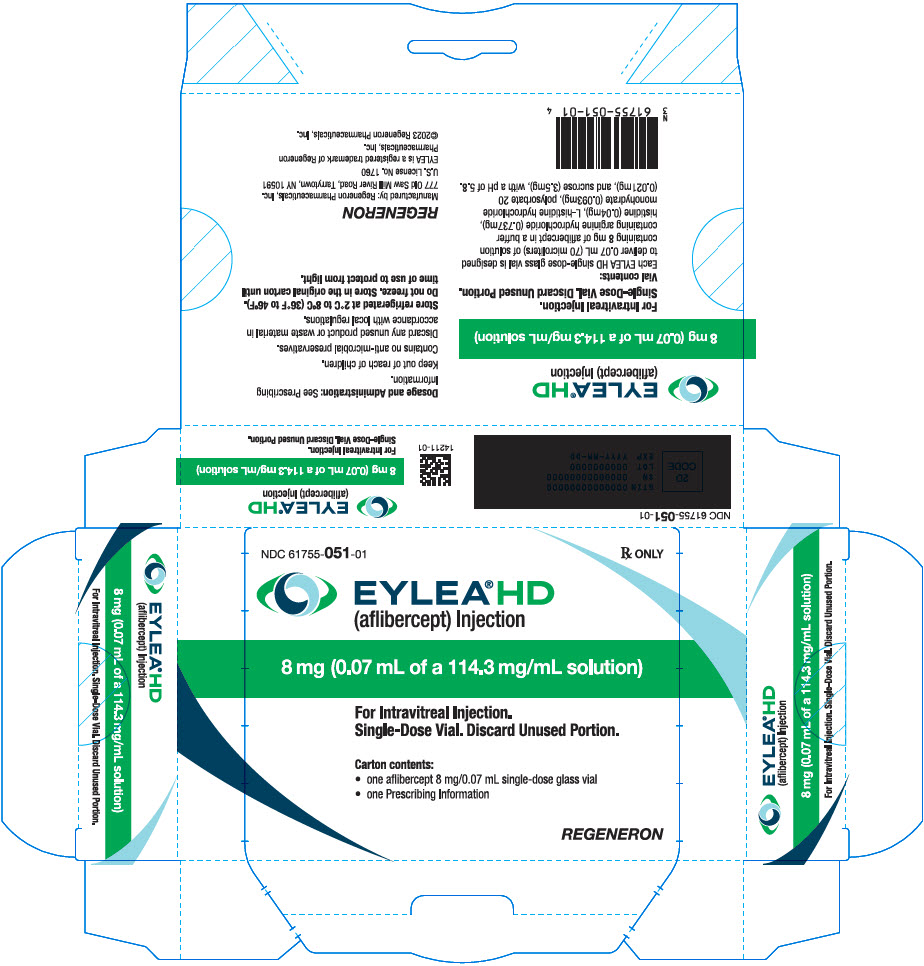
PRINCIPAL DISPLAY PANEL - 0.07 mL Vial Carton - 051
NDC: 61755-051-01
Rx ONLY
EYLEA®HD
(aflibercept) Injection8 mg (0.07 mL of a 114.3 mg/mL solution)
For Intravitreal Injection.
Single-Dose Vial. Discard Unused Portion.Carton contents:
- one aflibercept 8 mg/0.07 mL single-dose glass vial
- one Prescribing Information
REGENERON
-
INGREDIENTS AND APPEARANCE
EYLEA HD
aflibercept injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61755-050 Route of Administration INTRAVITREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aflibercept (UNII: 15C2VL427D) (aflibercept - UNII:15C2VL427D) aflibercept 8 mg in 0.07 mL Inactive Ingredients Ingredient Name Strength polysorbate 20 (UNII: 7T1F30V5YH) sucrose (UNII: C151H8M554) arginine hydrochloride (UNII: F7LTH1E20Y) histidine (UNII: 4QD397987E) HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61755-050-01 1 in 1 CARTON 08/18/2023 1 NDC: 61755-050-00 0.07 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package 2 NDC: 61755-050-51 1 in 1 CARTON 08/18/2023 2 NDC: 61755-050-50 0.07 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761355 08/18/2023 EYLEA HD
aflibercept injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61755-051 Route of Administration INTRAVITREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength aflibercept (UNII: 15C2VL427D) (aflibercept - UNII:15C2VL427D) aflibercept 8 mg in 0.07 mL Inactive Ingredients Ingredient Name Strength polysorbate 20 (UNII: 7T1F30V5YH) sucrose (UNII: C151H8M554) arginine hydrochloride (UNII: F7LTH1E20Y) histidine (UNII: 4QD397987E) HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61755-051-01 1 in 1 CARTON 08/18/2023 1 NDC: 61755-051-00 0.07 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 61755-051-51 1 in 1 CARTON 08/18/2023 2 NDC: 61755-051-50 0.07 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761355 08/18/2023 Labeler - Regeneron Pharmaceuticals, Inc (194873139) Establishment Name Address ID/FEI Business Operations Regeneron Pharmaceuticals, Inc 945589711 API MANUFACTURE(61755-050, 61755-051) , ANALYSIS(61755-050, 61755-051) , MANUFACTURE(61755-050, 61755-051)
Trademark Results [EYLEA HD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EYLEA HD 97264271 not registered Live/Pending |
Regeneron Pharmaceuticals, Inc. 2022-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.