CHOICE LIQUID ANTIBACTERIAL- parachlorometaxylenol liquid
Choice Liquid Antibacterial by
Drug Labeling and Warnings
Choice Liquid Antibacterial by is a Otc medication manufactured, distributed, or labeled by Inopak, Ltd., Inopak, Lt.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
- Warnings
- When using
- Stop using
- Keep away from Children
- Directions
- Inactive ingredients
-
Choice Liquid Antibacterial Soap Single Label
Rev. 1.01
Broad
Spectrum
Germ Control
Contains
.3%
P.C.M.X
Choice
LIQUID
ANTIBACTERIAL
SOAP
With Moisturizers
Drug Facts
Active Ingredient Purpose
Parachlorometaxylenol 0.3% w/w…. Antiseptic
Uses
Handwash to help reduce bacteria on the skin that
potentially can cause disease.
Recommended for repeated use.
Warnings
For external use only.
Keep out of eyes, ears, or mouth. In case of eye
contact, flush eyes with water.
Keep out of reach of children. If swallowed, get
medical help or contact a Poison Control Center
right away.
Directions
Wet hands with water and dispense sufficient
amount of product into cupped palm of hand.
Wash both hands thoroughly for 15 seconds.
Rinse under running water and dry thoroughly.
Inactive ingredients Citric Acid, Cocamide DEA, D&C Red 4, DMOM Hydantoin, Ethyl Alcohol, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, Sodium Chloride, Sodium Laureth Sulfate, Water.
Questions? Call 1-800-767-7725 or visit our website www.inopack.com

- Choice Case label
-
Choice Box front label
401-1-80
Drug Facts
Active Ingredient Purpose
Parachlorometaxylenol 0.3% w/w…. Antiseptic
Uses
Handwash to help reduce bacteria on the skin that
potentially can cause disease.
Recommended for repeated use.
Warnings
For external use only.
Keep out of eyes, ears, or mouth. In case of eye
contact, flush eyes with water.
Keep out of reach of children. If swallowed, get
medical help or contact a Poison Control Center
right away.
Directions
Wet hands with water and dispense sufficient
amount of product into cupped palm of hand.
Wash both hands thoroughly for 15 seconds.
Rinse under running water and dry thoroughly.
Inactive ingredients Citric Acid, Cocamide DEA, D&C Red 4, DMOM Hydantoin, Ethyl Alcohol, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, Sodium Chloride, Sodium Laureth Sulfate, Water.
Choice
LIQUID ANTIBACTERIAL SOAP
With Moisturizers
Contains
.3% P.C.M.X.
PN-5031-404UV-CH
800 ml

-
Choice box back label
Choice
LIQUID ANTIBACTERIAL SOAP
LIQUID ANTIBACTERIAL SOAP
Antibacterial soap can help reduce bacteria
on the hands that cause disease
pH balanced
Produced in an FDA registered facility
cGMP facility
Mild formula for multiple handwashing without stripping the skin of its natural oils
Broad
Spectrum
Germ
Control
MADE
IN THE
USA
Choice
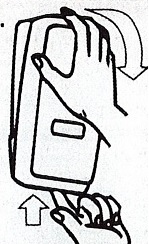
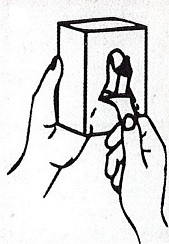
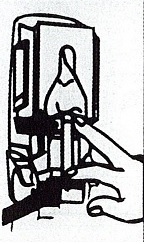
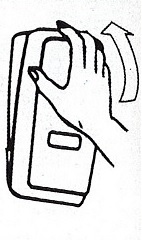
Dispenser Loading Instructions
1.
2.
3.
4.

-
INGREDIENTS AND APPEARANCE
CHOICE LIQUID ANTIBACTERIAL
parachlorometaxylenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58575-407 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID ACETATE (UNII: DSO12WL7AU) COCAMIDOPROPYL DIMETHYLAMINE (UNII: L36BM7DG2T) DMDM HYDANTOIN (UNII: BYR0546TOW) ALCOHOL (UNII: 3K9958V90M) FD&C RED NO. 4 (UNII: X3W0AM1JLX) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ISOPROPYL ALCOHOL (UNII: ND2M416302) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58575-407-01 3785 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2018 2 NDC: 58575-407-02 800 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2018 3 NDC: 58575-407-03 9600 mL in 1 CASE; Type 0: Not a Combination Product 01/01/2018 4 NDC: 58575-407-04 15140 mL in 1 CASE; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/2018 Labeler - Inopak, Ltd. (194718243) Establishment Name Address ID/FEI Business Operations Inopak, Lt. 194718243 manufacture(58575-407)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
