DDAVP- desmopressin acetate spray
DDAVP by
Drug Labeling and Warnings
DDAVP by is a Prescription medication manufactured, distributed, or labeled by Ferring Pharmaceuticals Inc., Ferring GmbH, Ferring International Center SA, Rechon Life Science AB, PolyPeptide Laboratories (Sweden) AB. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Rx only
-
DESCRIPTION
DDAVP® Rhinal Tube is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows:
Mol. wt. 1183.34
Empirical formula: C46H64N14O12S2∙C2H4O2∙3H2O
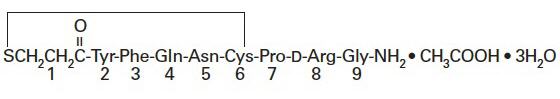
1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
DDAVP Rhinal Tube is provided as an aqueous solution for intranasal use.
Each mL contains: Desmopressin acetate 0.1 mg Chlorobutanol 5 mg Sodium Chloride 9 mg Hydrochloric acid to adjust pH to approximately 4 -
CLINICAL PHARMACOLOGY
DDAVP Rhinal Tube contains as active substance desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin. One mL (0.1 mg) of intranasal DDAVP (desmopressin acetate) has an antidiuretic activity of about 400 IU; 10 mcg of desmopressin acetate is equivalent to 40 IU.
- The biphasic half-lives for intranasal DDAVP were 7.8 and 75.5 minutes for the fast and slow phases, compared with 2.5 and 14.5 minutes for lysine vasopressin, another form of the hormone used in this condition. As a result, intranasal DDAVP provides a prompt onset of antidiuretic action with a long duration after each administration.
- The change in structure of arginine vasopressin to DDAVP has resulted in a decreased vasopressor action and decreased actions on visceral smooth muscle relative to the enhanced antidiuretic activity, so that clinically effective antidiuretic doses are usually below threshold levels for effects on vascular or visceral smooth muscle.
- DDAVP administered intranasally has an antidiuretic effect about one-tenth that of an equivalent dose administered by injection.
Human Pharmacokinetics
DDAVP is mainly excreted in the urine. A pharmacokinetic study conducted in healthy volunteers and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects in each group) receiving single dose desmopressin acetate (2 mcg) injection demonstrated a difference in DDAVP terminal half-life. Terminal half-life significantly increased from 3 hours in normal healthy patients to 9 hours in patients with severe renal impairment. (See CONTRAINDICATIONS.)
-
INDICATIONS AND USAGE
Central Cranial Diabetes Insipidus
DDAVP Rhinal Tube is indicated as antidiuretic replacement therapy in the management of central cranial diabetes insipidus and for management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. It is ineffective for the treatment of nephrogenic diabetes insipidus.
The use of DDAVP Rhinal Tube in patients with an established diagnosis will result in a reduction in urinary output with increase in urine osmolality and a decrease in plasma osmolality. This will allow the resumption of a more normal life-style with a decrease in urinary frequency and nocturia.
There are reports of an occasional change in response with time, usually greater than 6 months. Some patients may show a decreased responsiveness, others a shortened duration of effect. There is no evidence this effect is due to the development of binding antibodies but may be due to a local inactivation of the peptide.
Patients are selected for therapy by establishing the diagnosis by means of the water deprivation test, the hypertonic saline infusion test, and/or the response to antidiuretic hormone. Continued response to intranasal DDAVP can be monitored by urine volume and osmolality.
DDAVP is also available as a solution for injection when the intranasal route may be compromised. These situations include nasal congestion and blockage, nasal discharge, atrophy of nasal mucosa, and severe atrophic rhinitis. Intranasal delivery may also be inappropriate where there is an impaired level of consciousness. In addition, cranial surgical procedures, such as transsphenoidal hypophysectomy create situations where an alternative route of administration is needed as in cases of nasal packing or recovery from surgery.
-
CONTRAINDICATIONS
DDAVP Rhinal Tube is contraindicated in individuals with known hypersensitivity to desmopressin acetate or to any of the components of DDAVP Rhinal Tube.
DDAVP is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50 mL/min).
DDAVP is contraindicated in patients with hyponatremia or a history of hyponatremia.
-
WARNINGS
- For intranasal use only.
- DDAVP Rhinal Tube should only be used in patients where orally administered formulations are not feasible.
- Very rare cases of hyponatremia have been reported from world-wide postmarketing experience in patients treated with DDAVP (desmopressin acetate). DDAVP is a potent antidiuretic which, when administered, may lead to water intoxication and/or hyponatremia. Unless properly diagnosed and treated hyponatremia can be fatal. Therefore, fluid restriction is recommended and should be discussed with the patient and/or guardian. Careful medical supervision is required.
- When DDAVP is administered, in particular, in pediatric and geriatric patients, fluid intake should be adjusted downward in order to decrease the potential occurrence of water intoxication and hyponatremia (See PRECAUTIONS, Pediatric Use and Geriatric Use.) All patients receiving DDAVP therapy should be observed for the following signs or symptoms associated with hyponatremia: headache, nausea/vomiting, decreased serum sodium, weight gain, restlessness, fatigue, lethargy, disorientation, depressed reflexes, loss of appetite, irritability, muscle weakness, muscle spasms or cramps and abnormal mental status such as hallucinations, decreased consciousness and confusion. Severe symptoms may include one or a combination of the following: seizure, coma and/or respiratory arrest. Particular attention should be paid to the possibility of the rare occurrence of an extreme decrease in plasma osmolality that may result in seizures which could lead to coma.
- DDAVP should be used with caution in patients with habitual or psychogenic polydipsia who may be more likely to drink excessive amounts of water, putting them at greater risk of hyponatremia.
-
PRECAUTIONS
General
Intranasal DDAVP at high dosage has infrequently produced a slight elevation of blood pressure, which disappeared with a reduction in dosage. The drug should be used with caution in patients with coronary artery insufficiency and/or hypertensive cardiovascular disease because of possible rise in blood pressure.
DDAVP should be used with caution in patients with conditions associated with fluid and electrolyte imbalance, such as cystic fibrosis, heart failure and renal disorders because these patients are prone to hyponatremia.
Ensure that in children administration is under adult supervision in order to control the dose intake.
Rare severe allergic reactions have been reported with DDAVP. Anaphylaxis has been reported rarely with intravenous and intranasal administration of DDAVP.
Central Cranial Diabetes Insipidus
Since DDAVP Rhinal Tube is used intranasally, changes in the nasal mucosa such as scarring, edema, or other disease may cause erratic, unreliable absorption in which case intranasal DDAVP should not be used. For such situations, DDAVP Injection should be considered.
Laboratory Tests
Laboratory tests for following the patient with central cranial diabetes insipidus or post-surgical or head trauma-related polyuria and polydipsia include urine volume and osmolality. In some cases plasma osmolality measurements may be required.
Drug Interactions
Although the pressor activity of DDAVP is very low compared to the antidiuretic activity, use of large doses of intranasal DDAVP with other pressor agents should only be done with careful patient monitoring. The concomitant administration of drugs that may increase the risk of water intoxication with hyponatremia, (e.g., tricyclic antidepressants, selective serotonin re-uptake inhibitors, chlorpromazine, opiate analgesics, NSAIDs, lamotrigine and carbamazepine) should be performed with caution.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with DDAVP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy Category B
Fertility studies have not been done. Teratology studies in rats and rabbits at doses from 0.05 to 10 mcg/kg/day (approximately 0.1 times the maximum systemic human exposure in rats and up to 38 times the maximum systemic human exposure in rabbits based on surface area, mg/m2) revealed no harm to the fetus due to DDAVP. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Several publications of desmopressin acetate's use in the management of diabetes insipidus during pregnancy are available; these include a few anecdotal reports of congenital anomalies and low birth weight babies. However, no causal connection between these events and desmopressin acetate has been established. A fifteen year, Swedish epidemiologic study of the use of desmopressin acetate in pregnant women with diabetes insipidus found the rate of birth defects to be no greater than that in the general population; however the statistical power of this study is low. As opposed to preparations containing natural hormones, desmopressin acetate in antidiuretic doses has no uterotonic action and the physician will have to weigh the therapeutic advantages against the possible risks in each case.
Nursing Mothers
There have been no controlled studies in nursing mothers. A single study in postpartum women demonstrated a marked change in plasma, but little if any change in assayable DDAVP (desmopressin acetate) in breast milk following an intranasal dose of 10 mcg. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when DDAVP is administered to a nursing woman.
Pediatric Use
Central Cranial Diabetes Insipidus
DDAVP Rhinal Tube has been used in pediatric patients with diabetes insipidus. Use in infants and pediatric patients will require careful fluid intake restriction to prevent possible hyponatremia and water intoxication. Fluid restriction should be discussed with the patient and/or guardian. (See WARNINGS.) The dose must be individually adjusted to the patient with attention in the very young to the danger of an extreme decrease in plasma osmolality with resulting convulsions. Dose should start at 0.05 mL or less.
There are reports of an occasional change in response with time, usually greater than 6 months. Some patients may show a decreased responsiveness, others a shortened duration of effect. There is no evidence this effect is due to the development of binding antibodies but may be due to a local inactivation of the peptide.
Geriatric Use
Clinical studies of DDAVP Rhinal Tube did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. DDAVP is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50 mL/min). (See CLINICAL PHARMACOLOGY, Human Pharmacokinetics and CONTRAINDICATIONS.)
Use of DDAVP Rhinal Tube in geriatric patients will require careful fluid intake restrictions to prevent possible hyponatremia and water intoxication. Fluid restriction should be discussed with the patient. (See WARNINGS.)
-
ADVERSE REACTIONS
Infrequently, high dosages of intranasal DDAVP have produced transient headache and nausea. Nasal congestion, rhinitis and flushing have also been reported occasionally along with mild abdominal cramps. These symptoms disappeared with reduction in dosage. Nosebleed, sore throat, cough and upper respiratory infections have also been reported.
The following table lists the percent of patients having adverse experiences without regard to relationship to study drug from the pooled pivotal study data for nocturnal enuresis.
ADVERSE REACTION PLACEBO
(N=59)
%DDAVP
20 mcg
(N=60)
%DDAVP
40 mcg
(N=61)
%BODY AS A WHOLE Abdominal Pain 0 2 2 Asthenia 0 0 2 Chills 0 0 2 Headache 0 2 5 NERVOUS SYSTEM Dizziness 0 0 3 RESPIRATORY SYSTEM Epistaxis 2 3 0 Nostril Pain 0 2 0 Rhinitis 2 8 3 DIGESTIVE SYSTEM Gastrointestinal Disorder 0 2 0 Nausea 0 0 2 SPECIAL SENSES Conjunctivitis 0 2 0 Edema Eyes 0 2 0 Lachrymation Disorder 0 0 2 Post Marketing
There have been rare reports of hyponatremic convulsions associated with concomitant use with the following medications: oxybutinin and imipramine.
See WARNINGS for the possibility of water intoxication and hyponatremia.
-
OVERDOSAGE
Signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention. (See WARNINGS.) In case of overdosage, the dose should be reduced, frequency of administration decreased, or the drug withdrawn according to the severity of the condition. There is no known specific antidote for desmopressin acetate or DDAVP Rhinal Tube.
An oral LD50 has not been established. An intravenous dose of 2 mg/kg in mice demonstrated no effect.
-
DOSAGE AND ADMINISTRATION
Central Cranial Diabetes Insipidus
This drug is administered into the nose through a soft, flexible plastic rhinal tube which has four graduation marks on it that measure 0.2, 0.15, 0.1, 0.05 and 0.025 mL. DDAVP Rhinal Tube dosage must be determined for each individual patient and adjusted according to the diurnal pattern of response. Response should be estimated by two parameters: adequate duration of sleep and adequate, not excessive, water turnover. Patients with nasal congestion and blockage have often responded well to intranasal DDAVP. The usual dosage range in adults is 0.1 to 0.4 mL daily, either as a single dose or divided into two or three doses. Most adults require 0.2 mL daily in two divided doses. The morning and evening doses should be separately adjusted for an adequate diurnal rhythm of water turnover. For children aged 3 months to 12 years, the usual dosage range is 0.05 to 0.3 mL daily, either as a single dose or divided into two doses. About 1/4 to 1/3 of patients can be controlled by a single daily dose of DDAVP administered intranasally. Fluid restriction should be observed. (See WARNINGS, PRECAUTIONS, Pediatric Use and Geriatric Use.)
Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY, Human Pharmacokinetics, CONTRAINDICATIONS, and PRECAUTIONS, Geriatric Use.)
-
HOW SUPPLIED
DDAVP Rhinal Tube is available in a 2.5 mL bottle, packaged with two rhinal tube applicators per carton (NDC: 55566-2400-0). Also available in a 5.0 mL pump bottle with spray pump delivery 50 doses of 10 mcg (NDC: 55566-2500-0).
- SPL UNCLASSIFIED SECTION
-
PATIENT INSTRUCTION GUIDE
DDAVP ® Rhinal Tube
(desmopressin acetate)Ensure that in children administration is under adult supervision in order to control the dose intake.
If you accidentally deliver/administer too much of a dose, immediately telephone your doctor or a certified Regional Poison Center for advice. Possible signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention.
- Pull plastic tag on neck of bottle.
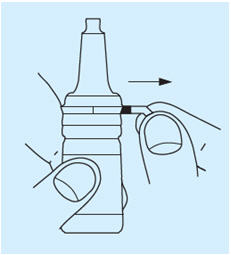
- Break security seal and remove plastic cap.
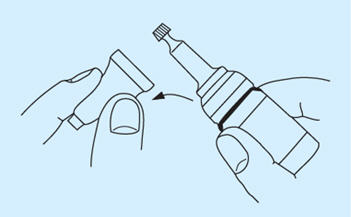
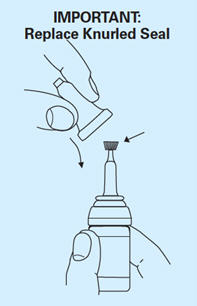
- Twist off the small knurled seal from the dropper. Use the same seal reversed to prevent subsequent leakage, especially if the bottle is not stored upright.
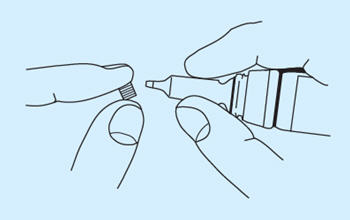
- The drug is administered by a soft, flexible, plastic rhinal tube which has dose marks at 0.2, 0.15, 0.1, 0.05 and 0.025 mL. Take the arrow-marked part of the tube in one hand and place the fingers of the other hand around the cylindrical part of the closure. Insert the top of the dropper in a downward position into the arrow-marked end of the tube and squeeze the dropper until the solution has reached the desired calibration mark. The dose is measured from the arrow-marked end of the tube to the appropriate calibration. Disconnect the tube from the bottle by withdrawing the bottle quickly downwards. In order to prevent air bubbles from forming in the tube, maintain constant pressure on the dropper. If difficulty is experienced in filling the tube, a diabetic or tuberculin syringe may be used to draw up the dose and load the tube.
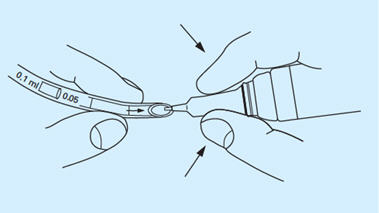
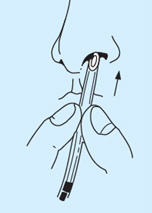
- Hold the tube with the fingers approximately ¾ inch from the end and insert into a nostril until the tips of the fingers reach the nostril.
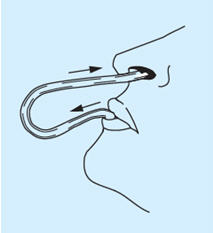
- Put the other end of the tube into the mouth. Hold the breath, tilt the head back and then blow with a short, strong puff through the tube so that the solution reaches the right place in the nasal cavity. Through this procedure, medication is limited to the nasal cavity and the preparation does not pass down into the throat.
In very young patients, it may be necessary for an adult to blow the solution into the child's nose. In such cases, the tube will not need to be put into the nose as far as in the older child or adult. The tube should be placed in the nose gently just far enough so that the solution does not run out. A baby must be held firmly and securely.
- After use, reseal dropper tip and close the bottle with the plastic cap. Wash the tube in water and shake thoroughly, until no more water is left. The tube can then be used for the next application.
Store refrigerated 2 to 8°C (36 to 46°F). When traveling, closed bottles will maintain stability for 3 weeks when stored at controlled room temperature, 20 to 25°C (68 to 77°F).
Manufactured for:
Ferring Pharmaceuticals Inc.
Parsippany, NJ 07054 USA
Origin SwedenRev. 04/2015
2009054185
-
PRINCIPAL DISPLAY PANEL - 2.5 mL Bottle Carton
NDC: 55566-2400-0
DDAVP®
Rhinal
Tube
desmopressin
acetate10 mcg/0.1 mL
FOR INTRANASAL USE ONLY
2.5 mL Bottle
Rx ONLY
FERRING
PHARMACEUTICALS
-
INGREDIENTS AND APPEARANCE
DDAVP
desmopressin acetate sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55566-2400 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESMOPRESSIN ACETATE (UNII: XB13HYU18U) (DESMOPRESSIN - UNII:ENR1LLB0FP) DESMOPRESSIN ACETATE 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL CHLOROBUTANOL (UNII: HM4YQM8WRC) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55566-2400-0 1 in 1 CARTON 02/21/1978 1 2.5 mL in 1 BOTTLE, PUMP; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017922 02/21/1978 Labeler - Ferring Pharmaceuticals Inc. (103722955) Establishment Name Address ID/FEI Business Operations Ferring GmbH 328609615 manufacture(55566-2400) , analysis(55566-2400) , pack(55566-2400) , label(55566-2400) Establishment Name Address ID/FEI Business Operations Ferring International Center SA 481210362 pack(55566-2400) Establishment Name Address ID/FEI Business Operations Rechon Life Science AB 775207769 manufacture(55566-2400) , analysis(55566-2400) , pack(55566-2400) , label(55566-2400) Establishment Name Address ID/FEI Business Operations PolyPeptide Laboratories (Sweden) AB 356580779 API MANUFACTURE(55566-2400) , ANALYSIS(55566-2400)
Trademark Results [DDAVP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DDAVP 98511956 not registered Live/Pending |
Ferring B.V. 2024-04-22 |
 DDAVP 74734670 1997079 Live/Registered |
AVENTISUB LLC 1995-09-27 |
 DDAVP 73399946 1386510 Dead/Cancelled |
ARMOUR PHARMACEUTICAL COMPANY 1982-10-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.