PHENOBARBITAL WITH BELLADONNA ALKALOIDS ELIXIR
PHENOBARBITAL WITH BELLADONNA ALKALOIDS by
Drug Labeling and Warnings
PHENOBARBITAL WITH BELLADONNA ALKALOIDS by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PHENOBARBITAL WITH BELLADONNA ALKALOIDS- phenobarbital with belladonna alkaloids elixir
Bryant Ranch Prepack
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
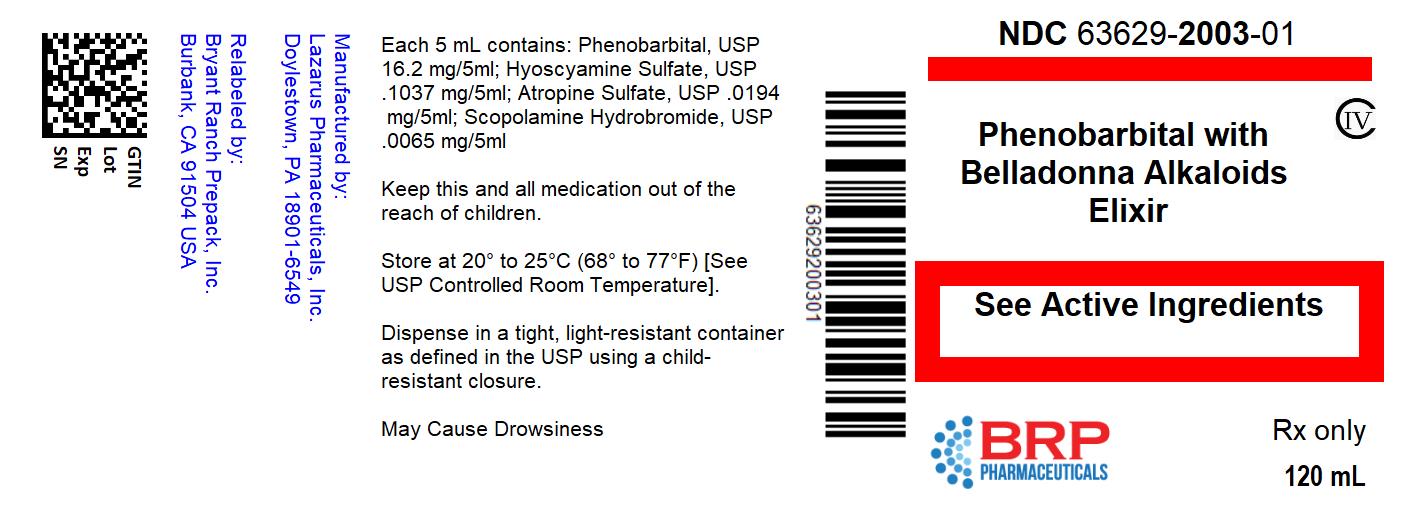
PHENOBARBITAL WITH BELLADONNA ALKALOIDS ELIXIR
DESCRIPTION:
Each 5 mL (teaspoonful) of elixir contains:
Phenobarbital, USP ................................................... 16.2 mg
Hyoscyamine Sulfate, USP ................................... 0.1037 mg
Atropine Sulfate, USP ........................................... 0.0194 mg
Scopolamine Hydrobromide, USP ......................... 0.0065 mg
CLINICAL PHARMACOLOGY:
This drug combination provides natural belladonna alkaloids in a specific, fixed ratio combined with phenobarbital to provide peripheral anticholinergic/antispasmodic action and mild sedation.
INDICATIONS AND USAGE:
Possibly effective for use as adjunctive therapy in the treatment of irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis. May also be useful as adjunctive therapy in the treatment of duodenal ulcer.
IT HAS NOT BEEN SHOWN CONCLUSIVELY WHETHER ANTICHOLINERGIC/ANTISPASMODIC DRUGS AID IN THE HEALING OF A DUODENAL ULCER, DECREASE THE RATE OF RECURRENCES OR PREVENT COMPLICATIONS.
CONTRAINDICATIONS:
Glaucoma, obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (as in achalasia, pyloroduodenal stenosis, etc.); paralytic ileus, intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis especially if complicated by toxic megacolon; myasthenia gravis; hiatal hernia associated with reflux esophagitis.
Phenobarbital with Belladonna Alkaloids Elixir is contraindicated in patients with known hypersensitivity to any of the ingredients. Phenobarbital is contraindicated in acute intermittent porphyria and in those patients in whom phenobarbital produces restlessness and/or excitement.
WARNINGS:
In the presence of a high environmental temperature, heat prostration can occur with belladonna alkaloids (fever and heatstroke due to decreased sweating). Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful.
Phenobarbital with Belladonna Alkaloids Elixir may produce drowsiness or blurred vision. The patient should be warned, should these occur, not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery, and not to perform hazardous work.
Phenobarbital may decrease the effect of anticoagulants, and necessitate larger doses of the anticoagulant for optimal effect. When the phenobarbital is discontinued, the dose of the anticoagulant may have to be decreased. Phenobarbital may be habit forming and should not be administered to individuals known to be addiction prone or to those with a history of physical and/or psychological dependence upon drugs. Since barbiturates are metabolized in the liver, they should be used with caution and initial doses should be small in patients with hepatic dysfunction.
PRECAUTIONS:
General
Use with caution in patients with: autonomic neuropathy, hepatic or renal disease, hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, tachycardia, and hypertension. Belladonna alkaloids may produce a delay in gastric emptying (antral stasis) which would complicate the management of gastric ulcer. Theoretically, with overdosage, a curare-like action may occur.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy Category C
Animal reproduction studies have not been conducted with Phenobarbital with Belladonna Alkaloids Elixir. It is not known whether Phenobarbital with Belladonna Alkaloids Elixir can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Phenobarbital with Belladonna Alkaloids Elixir should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS:
Adverse reactions may include xerostomia, urinary hesitancy and retention; blurred vision; tachycardia; palpitation; mydriasis; cycloplegia; increased ocular tension; loss of taste sense; headache; nervousness; drowsiness; weakness; dizziness; insomnia; nausea; vomiting; impotence; suppression of lactation; constipation; bloated feeling; musculoskeletal pain; severe allergic reaction or drug idiosyncrasies, including anaphylaxis, urticaria and other dermal manifestations; and decreased sweating. Elderly patients may react with symptoms of excitement, agitation, drowsiness, and other untoward manifestations to even small doses of the drug. Phenobarbital may produce excitement in some patients, rather than a sedative effect. In patients habituated to barbiturates, abrupt withdrawal may produce delirium or convulsions.
To report SUSPECTED ADVERSE REACTIONS, contact Lazarus Pharmaceuticals, Inc. at 1-888-296-9383 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION:
The dosage of Phenobarbital with Belladonna Alkaloids Elixir should be adjusted to the needs of the individual patient to assure symptomatic control with a minimum of adverse effects.
OVERDOSAGE:
The signs and symptoms of overdose are headache, nausea, vomiting, blurred vision, dilated pupils, hot and dry skin, dizziness, dryness of the mouth, difficulty in swallowing, CNS stimulation. Treatment should consist of gastric lavage, emetics and activated charcoal. If indicated, parenteral cholinergic agents such as physostigmine or bethanechol chloride, should be added.
| PHENOBARBITAL WITH BELLADONNA ALKALOIDS
phenobarbital with belladonna alkaloids elixir |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-2003) , RELABEL(63629-2003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.