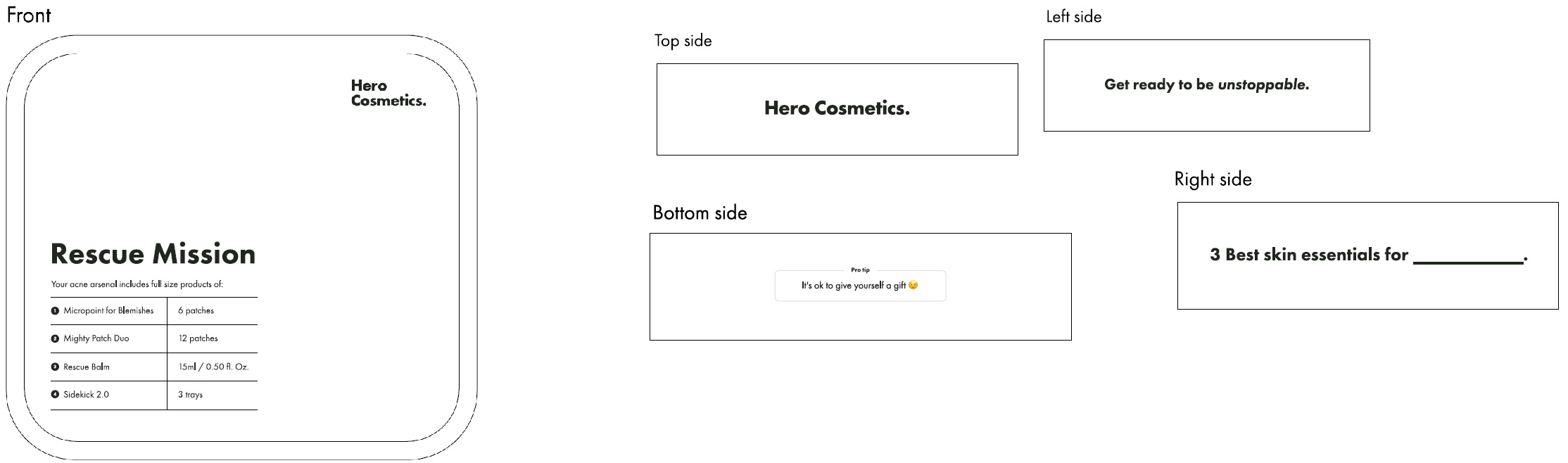
Hero Cosmetics Rescue Mission Holiday Kit
Hero Cosmetics Rescue Mission Holiday Kit by
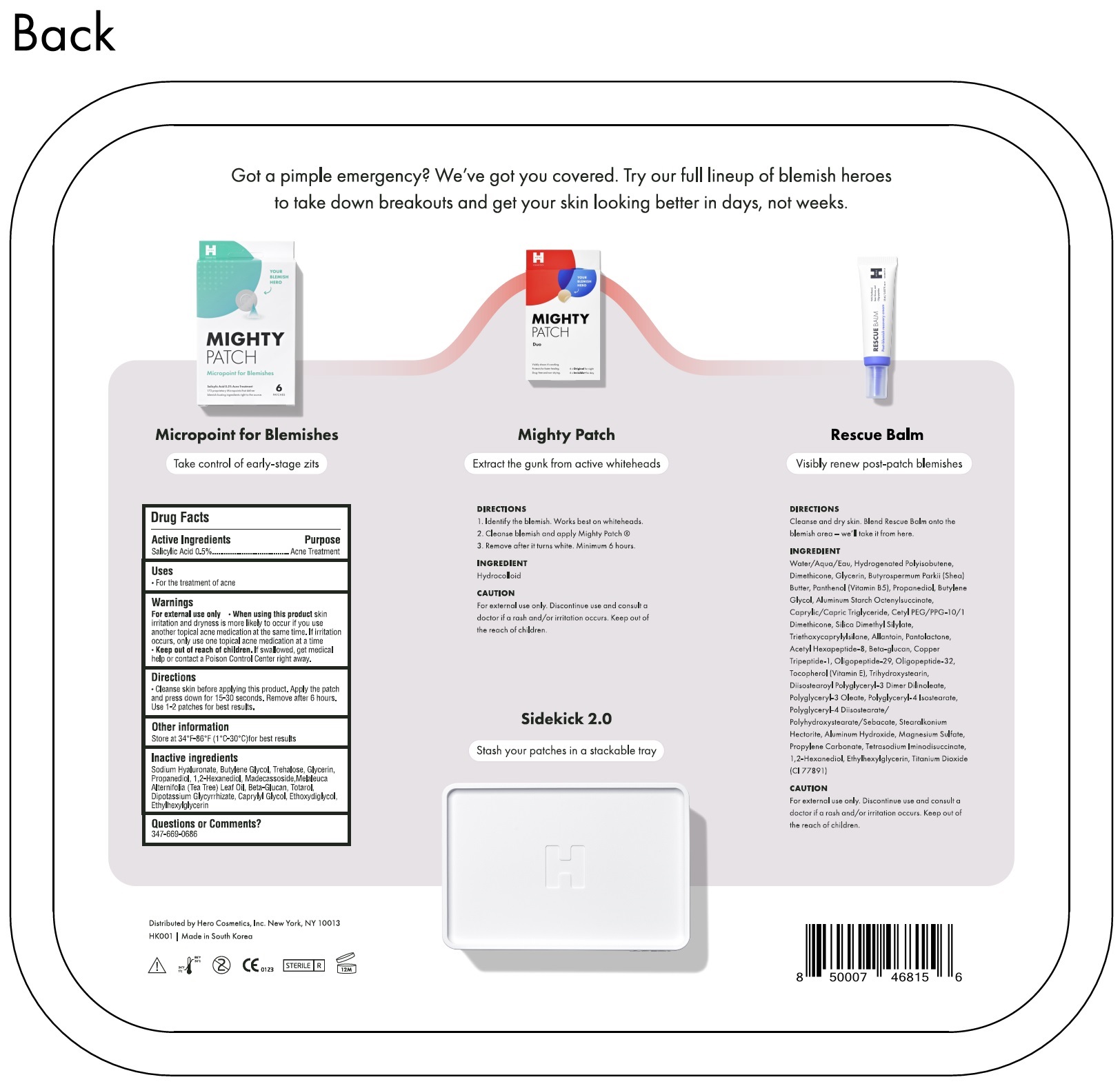
Drug Labeling and Warnings
Hero Cosmetics Rescue Mission Holiday Kit by is a Otc medication manufactured, distributed, or labeled by Hero Cosmetics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HERO COSMETICS RESCUE MISSION HOLIDAY KIT- salicylic acid
Hero Cosmetics, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hero Cosmetics Rescue Mission Holiday Kit
Warnings
For external use only
Directions
Clean the skin before applying this product. Apply the patch and press down for 15-30 seconds. Remove after 6 hours. Use 1-2 patches for best results.v
| HERO COSMETICS RESCUE MISSION HOLIDAY KIT
salicylic acid kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Hero Cosmetics, Inc. (053668306) |
Revised: 7/2022
Document Id: e326ca09-d53c-d84e-e053-2995a90af9c1
Set id: 8755207e-1d5d-4007-9c75-2cf4b30166e6
Version: 3
Effective Time: 20220706
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.