Joint and Muscle Cream by HTO Nevada Inc. DRUG FACTS
Joint and Muscle Cream by
Drug Labeling and Warnings
Joint and Muscle Cream by is a Otc medication manufactured, distributed, or labeled by HTO Nevada Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
JOINT AND MUSCLE CREAM- menthol cream
HTO Nevada Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
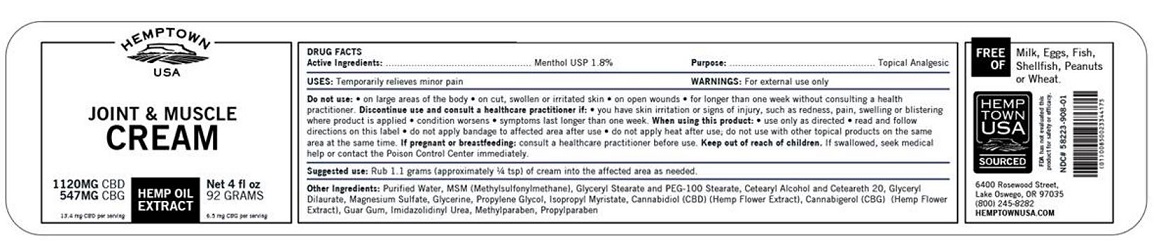
DRUG FACTS
Do not use:
- on large areas of the body
- on cut, swollen or irritated skin
- on open wounds
- for longer than one week without consulting a health practitioner.
Discontinue use and consult a healthcare practitioner if:
- you have skin irritation or signs of injury, such as redness, pain, swelling or blistering where product is applied
- condition worsens
- symptoms last longer than one week
When using this product:
- use only as directed
- read and follow directions on this label
- do not apply bandage to affected area after use
- do not apply heat after use; do not use with other topical products on the same area at the same time.
Keep out of reach of children.
If swallowed, seek medical help or contact the Poison Control Center immediately.
Other Ingredients: Purified Water, MSM (Methylsulfonymethane), Glyceryl Stearate and PEG-100 Stearate, Cetearyl Alcohol and Ceteareth 20, Glyceryl Dilaurate, Magnesium Sulfate, Glycerine, Propylene Glycol, Cannabidiol (CBD) (Hemp Flower Extract), Isopropyl Mytistate, Cannabigerol (CBG) (Hemp Flower Extract), Guar Gum, Imidazolidinyl Urea, Methylparaben, Propylparaben
PRINCIPAL DISPLAY PANEL
HEMPTOWN
USA
JOINT & MUSCLE
CREAM
1128MG CBD
552MG CBG
HEMP OIL
EXTRACT
Net 4 fl oz
92 GRAMS
13.5 mg CBD per serving
6.6 mg CBG per serving
FREE OF Milk, Eggs, Fish, Shellfish, Peanuts or Wheat.
HEMP
TOWN
USA
SOURCED
NDC# 58223-908-01
The FDA has not evaluated this
product for safety or efficacy
6400 Rosewood Street
Lake Oswego, OR 97035
(800) 245-8282
HEMPTTOWNUSA.COM
| JOINT AND MUSCLE CREAM
menthol cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - HTO Nevada Inc. (117115846) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.