NORTHSIDE HOSPITAL AMENITY KIT- alcohol and sodium monofluorophospate kit
Northside Hospital Amenity Kit by
Drug Labeling and Warnings
Northside Hospital Amenity Kit by is a Otc medication manufactured, distributed, or labeled by ASP Global, LLc, Shengzhou Kingbird Travel Products Co., Ltd., Colgate-Palmolive (Thailand) LTD, Nantong Health & Beyond Hygienic Products Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician children 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician - Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 53 mL Bottle Label
NORTHSIDE HOSPITAL
Instant Hand Antiseptic
62% w/w Ethyl AlcoholCreating a Healing Enviroment
We provide our patients with the essentials
they need to stay well rested.
This is an important part of healing,
both here and at home.1.9 FL OZ / 53 mL
Distributed by:
ASP Global, LLC
3450 Atlanta Industrial Parkway
Atlanta, GA 30331Rev 12-2017

- PRINCIPAL DISPLAY PANEL - 10 g Tube Label
-
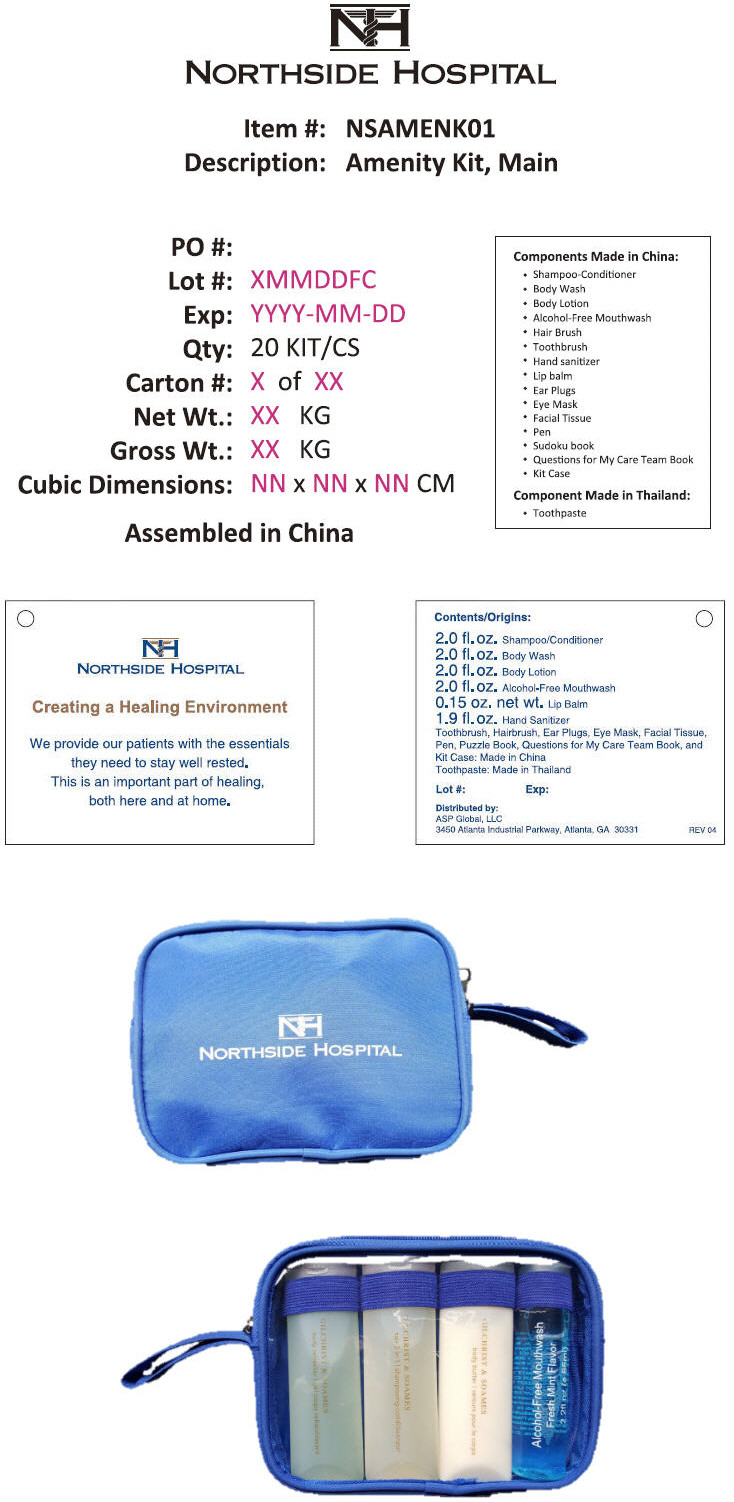
PRINCIPAL DISPLAY PANEL - Kit Label
NORTHSIDE HOSPITAL
Item #: NSAMENK01
Description: Amenity Kit, MainPO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 20 KIT/CS
Carton #: X of XX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: NN x NN x NN CMAssembled in China
Components Made in China:
- Shampoo-Conditioner
- Body Wash
- Body Lotion
- Alcohol-Free Mouthwash
- Hair Brush
- Toothbrush
- Hand sanitizer
- Lip balm
- Ear Plugs
- Eye Mask
- Facial Tissue
- Pen
- Sudoku book
- Questions for My Care Team Book
- Kit Case
Component Made in Thailand:
- Toothpaste
NORTHSIDE HOSPITAL
Creating a Healing Environment
We provide our patients with the essentials
they need to stay well rested.
This is an important part of healing,
both here and at home.Contents/Origins:
2.0 fl.oz. Shampoo/Conditioner
2.0 fl.oz. Body Wash
2.0 fl.oz. Body Lotion
2.0 fl.oz. Alcohol-Free Mouthwash
0.15 oz. net wt. Lip Balm
1.9 fl.oz. Hand Sanitizer
Toothbrush, Hairbrush, Ear Plugs, Eye Mask, Facial Tissue,
Pen, Puzzle Book, Questions for My Care Team Book, and
Kit Case: Made in China
Toothpaste: Made in ThailandLot #:
Exp:Distributed by:
ASP Global, LLC
3450 Atlanta Industrial Parkway, Atlanta, GA 30331REV 04
NORTHSIDE HOSPITAL
-
INGREDIENTS AND APPEARANCE
NORTHSIDE HOSPITAL AMENITY KIT
alcohol and sodium monofluorophospate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59448-200 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59448-200-00 1 in 1 BAG 01/18/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 53 mL Part 2 1 TUBE 10 g Part 3 1 TUBE 59 mL Part 4 1 TUBE 59 mL Part 5 1 TUBE 59 mL Part 6 1 TUBE 4.25 g Part 7 1 BOTTLE, PLASTIC 65 mL Part 1 of 7 INSTANT HAND ANTISEPTIC
alcohol gelProduct Information Item Code (Source) NDC: 59448-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) Alcohol 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Aloe Vera Leaf (UNII: ZY81Z83H0X) Glycerin (UNII: PDC6A3C0OX) Propylene Glycol (UNII: 6DC9Q167V3) Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Trolamine (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59448-001-02 53 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333E 12/18/2015 Part 2 of 7 COLGATE ANTICAVITY
sodium monofluorophosphate paste, dentifriceProduct Information Item Code (Source) NDC: 42555-060 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Monofluorophosphate (UNII: C810JCZ56Q) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 7.6 mg in 1 g Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Water (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Sodium Lauryl Sulfate (UNII: 368GB5141J) Sodium Pyrophosphate (UNII: O352864B8Z) Saccharin Sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42555-060-45 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 06/04/2009 Part 3 of 7 GILCHRIST AND SOAMES BODY BUTTER
cleansing (cold creams, cleansing lotions, liquids, and pads)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) INGR BENZYL ALCOHOL (UNII: LKG8494WBH) INGR PEG-100 STEARATE (UNII: YD01N1999R) INGR CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) INGR DEHYDROACETIC ACID (UNII: 2KAG279R6R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 07/01/2013 Part 4 of 7 GILCHRIST AND SOAMES HAIR 2 IN 1
shampoos (non-coloring)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR COCO MONOETHANOLAMIDE (UNII: C80684146D) INGR POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 2600000 MW) (UNII: U1G23TFV1K) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR MAGNESIUM NITRATE (UNII: 77CBG3UN78) INGR METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) INGR MAGNESIUM CHLORIDE (UNII: 02F3473H9O) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR FD&C RED NO. 4 (UNII: X3W0AM1JLX) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 07/01/2013 Part 5 of 7 GILCHRIST AND SOAMES BODY REFRESHER--BODY WASH
skin freshenersProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 2600000 MW) (UNII: U1G23TFV1K) INGR COCO MONOETHANOLAMIDE (UNII: C80684146D) INGR MAGNESIUM NITRATE (UNII: 77CBG3UN78) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) INGR MAGNESIUM CHLORIDE (UNII: 02F3473H9O) INGR METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR FD&C RED NO. 4 (UNII: X3W0AM1JLX) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color GREEN Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 07/01/2013 Part 6 of 7 NORTHSIDE HOSPITAL LIP BALM
lipstickProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ETHYLHEXYL PALMITATE (UNII: 2865993309) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR POLYISOBUTYLENE (2300 MW) (UNII: DSQ2V1DD1K) INGR YELLOW WAX (UNII: 2ZA36H0S2V) INGR HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) INGR SHEANUT OIL (UNII: O88E196QRF) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4.25 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 11/01/2016 Part 7 of 7 ASP GLOBAL, LLC ALCOHOL-FREE
mouthwashes and breath fresheners (liquids and sprays)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) INGR SACCHARIN SODIUM (UNII: SB8ZUX40TY) INGR CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 65 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 07/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/18/2016 Labeler - ASP Global, LLc (080361159) Establishment Name Address ID/FEI Business Operations Shengzhou Kingbird Travel Products Co., Ltd. 560219293 PACK(59448-200) , LABEL(59448-200) , REPACK(59448-200) Establishment Name Address ID/FEI Business Operations Colgate-Palmolive (Thailand) LTD 672044552 MANUFACTURE(59448-200) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 MANUFACTURE(59448-200)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
