4293 FIRST AID KIT- 4293 first aid kit
4293 First Aid Kit by
Drug Labeling and Warnings
4293 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
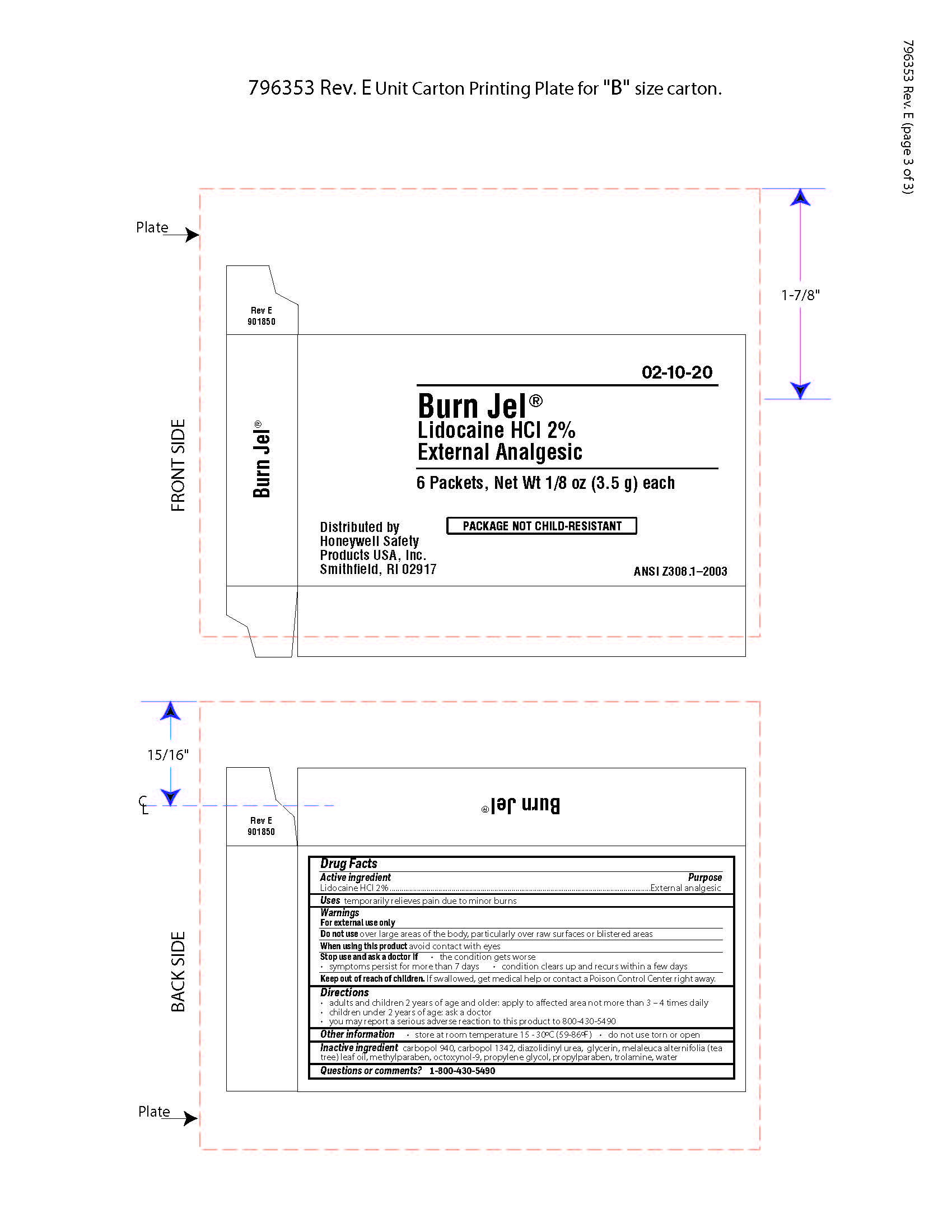
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn JEl Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
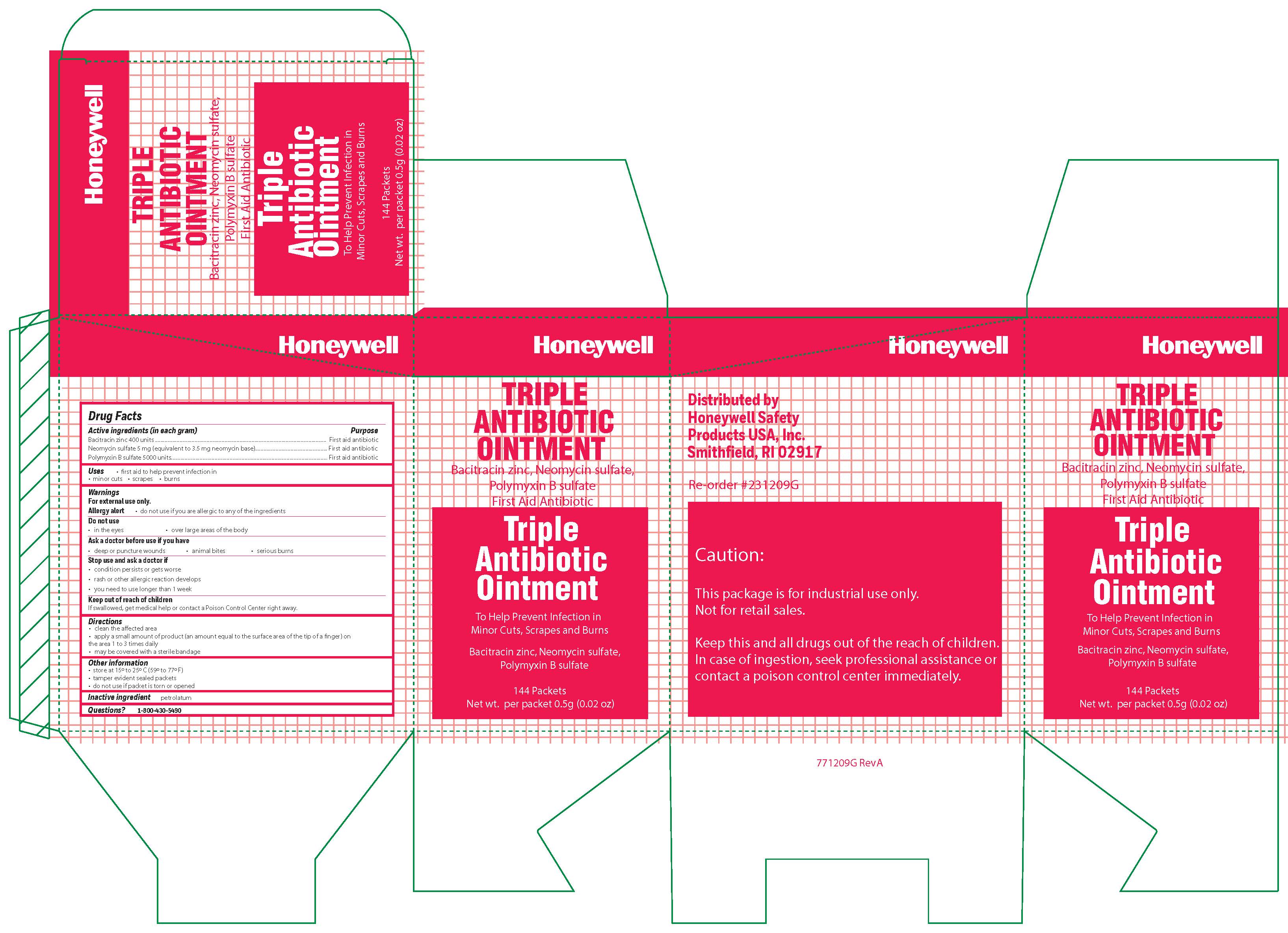
- Triple Active ingredient
- Triple Purpose
- Triple Uses
-
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
- Triple Questions?
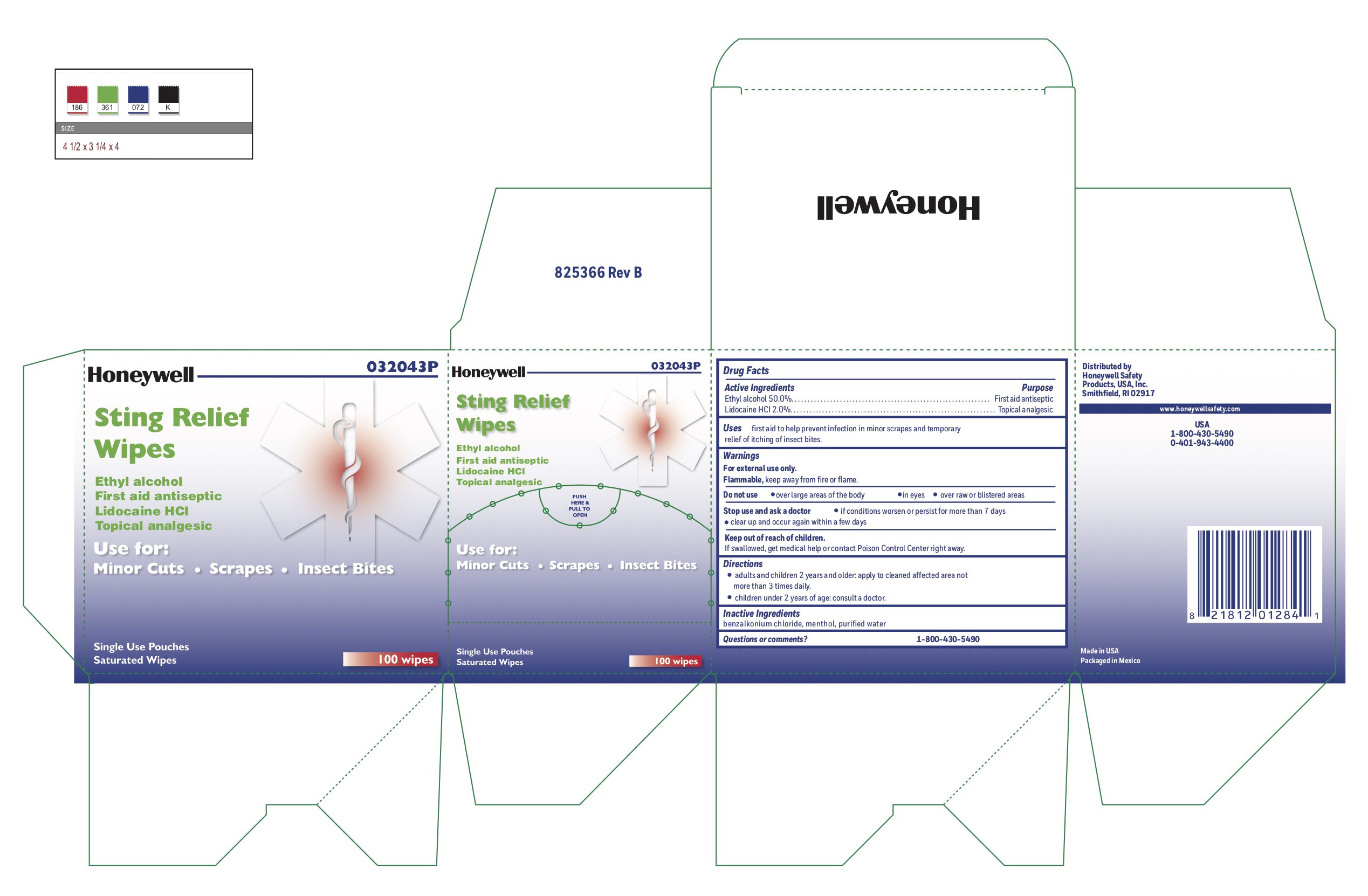
- Sting Relief Active ingredient (in each wipe)
- Sting Relief Purposse
- Sting Relief Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting Relief Questions or Comments?
- Eyewash Active ingredient
- Eyewassh Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyeash Directions
- Eyewash Inactive ingredients
- Eyeash Questions
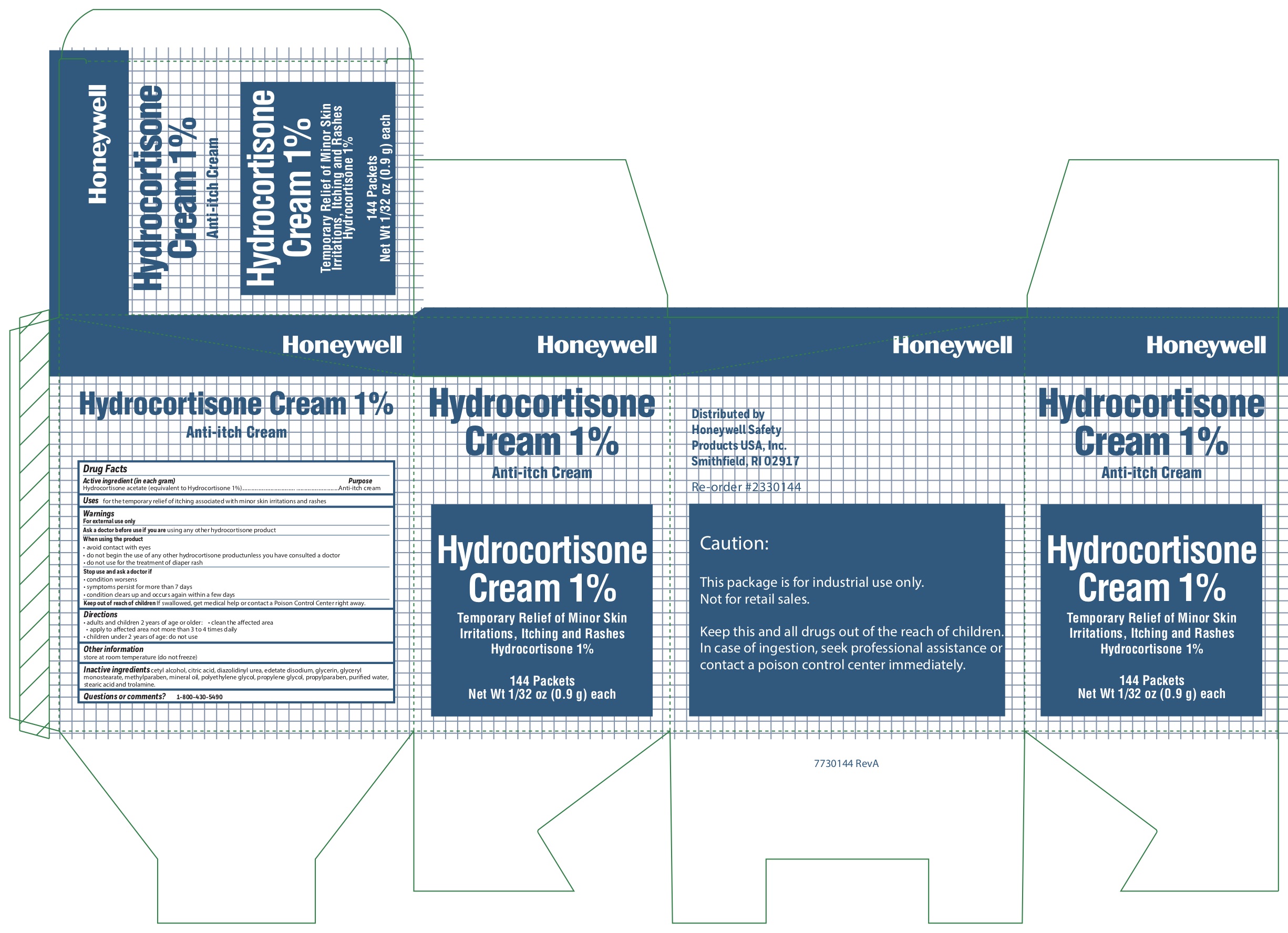
- Hydrocortisone Active ingredient (in each gram)
- Hydrcortisone Purpose
- Hydrocortisone Uses
-
Hydrocortisone
Warnings
For external use onlyWhen using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
- Hydrocortisone Directions
- Hydrocortisone Other information
- Hydrocortisone Inactive ingredients
- Hydrocortisone Questions or Comments?
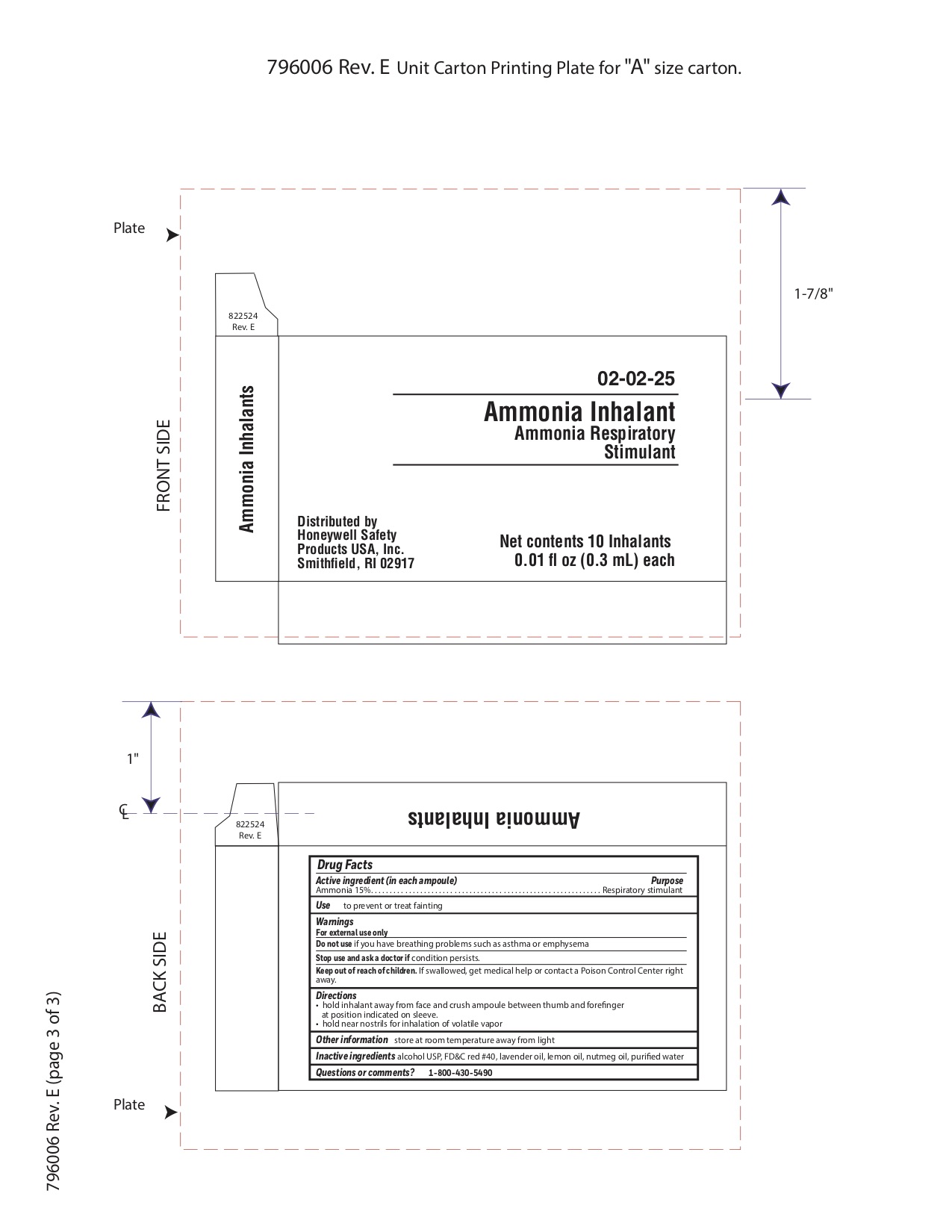
- Ammonia Active ingredient
- Ammonia Purpose
- Ammonia Uses
- Ammonia Warnings
- Ammonia Directions
- Ammonia Other information
- Ammonia Inactive ingredients
- Ammonia Questions or Comments?
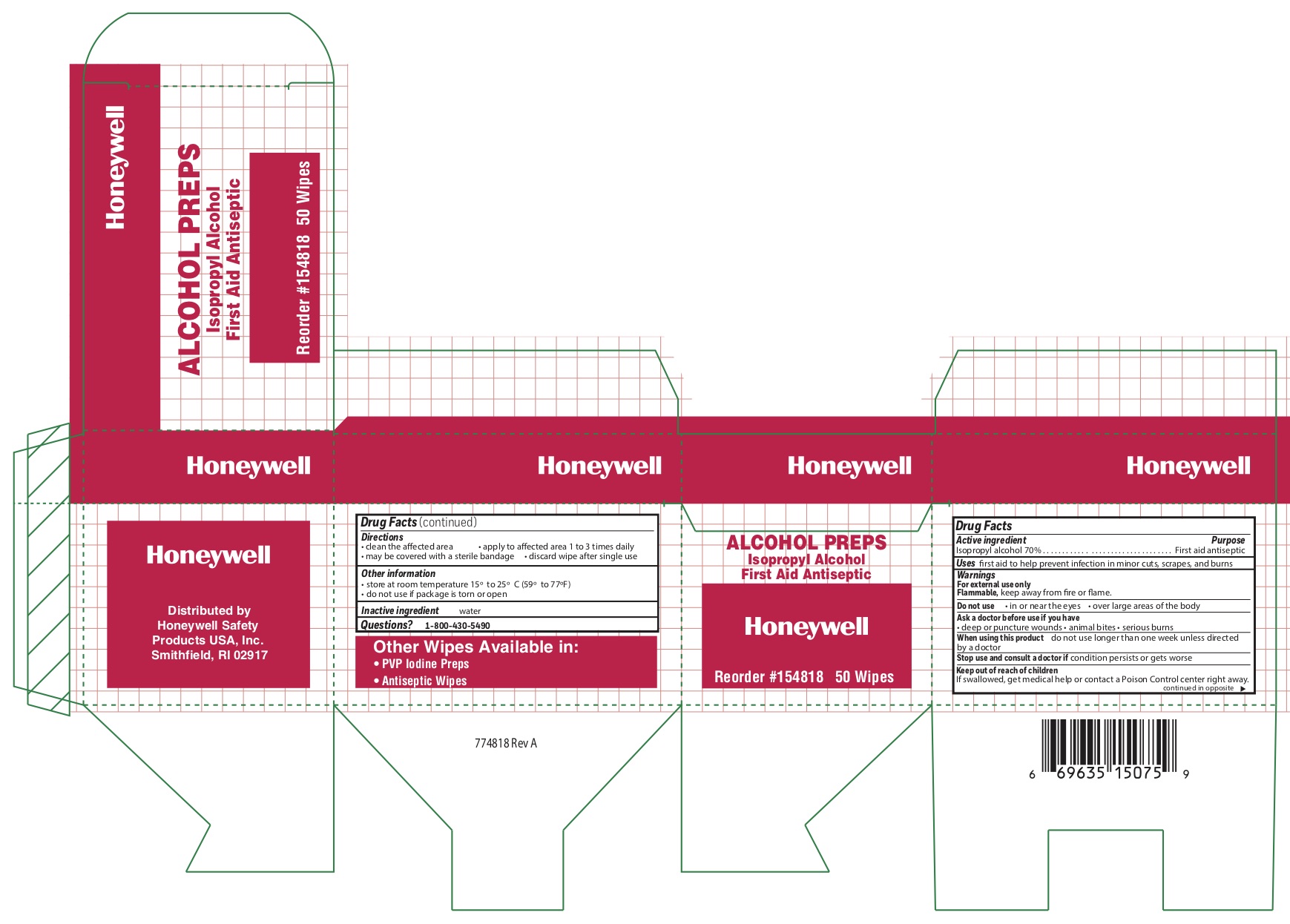
- Alcohol Active ingredient
- Alcohol Purpose
- Alcohol Uses
- Alcohol Warnings
- Alcohol Directions
- Alcohol Other information
- Alcohol Inactive ingredient
- Alcohol Questions
- PVP Active ingredient
- PVP Purpose
- PVP Uses
- PVP Warnings
- PVP Directions
- PVP Other information
- PVP Inactive ingredients
-
4293
SF00004080 Kit Contents
1 FNGRTIP-5 PER, KNCKL BDG-4 PER
1 TRIPLE ANTIBIOTIC 10 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 TRIANGULAR BDG, NON-STERILE
1 FORCEPS & SCISSORS, 1 EA
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
1 INSTANT COLD PACK 4" X 6"
1 ADH BDG W/NOADHR PAD,1X3 32PER
1 BURN JEL 1/8 OZ, 6 PER
1 ALCOHOL PREP PADS 10P
1 HYDROCORTISON,1.O%,1/32 OZ,10P
1 PVP IODINE WIPES 10 PER
1 STING RELIEF WIPES 10 PER BOX
1 ADH TAPE W/P 1/2"X 2 1/2 YD
1 FIRST AID GUIDE ASHI
1 1 OZ, BUFF EYEWASH
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 24 UN WHITE 01
- Burn Jel Principal Display Panel
- Triple Principal Display Panel
- Sting Relief Principal Display Panel
- Eyewash Principal Display Panel
- Hydrocortisone Principal Display Panel
- Ammonia Principal Display Panel
- Alcohol Principal Display Panel
- PVP Principal Display Panel
- 4293 Kit Label SF-00004080
-
INGREDIENTS AND APPEARANCE
4293 FIRST AID KIT
4293 first aid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4293 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4293-01 1 in 1 KIT 09/13/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKET 21 g Part 2 1 BOTTLE 30 mL Part 3 10 PACKET 9 g Part 4 10 POUCH 4 mL Part 5 10 POUCH 4 mL Part 6 10 PACKET 9 g Part 7 10 AMPULE 3 mL Part 8 10 POUCH 3 mL Part 9 10 PACKET 9 g Part 1 of 9 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) DIPROPYLENE GLYCOL (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/19/2018 Part 2 of 9 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 3 of 9 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC: 0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 4 of 9 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 5 of 9 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0733-00 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Part 6 of 9 HYDROCORTISONE
anti-itch cream ointmentProduct Information Item Code (Source) NDC: 0498-0800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TROLAMINE (UNII: 9O3K93S3TK) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0800-34 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/06/2013 10/15/2019 Part 7 of 9 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC: 0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 8 of 9 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NONOXYNOL-9 (UNII: 48Q180SH9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 9 of 9 HYDROCORTISONE
anti-itch creamProduct Information Item Code (Source) NDC: 0498-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TROLAMINE (UNII: 9O3K93S3TK) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/13/2018 Labeler - Honeywell Safety Products USA, Inc. (079287321) Registrant - Honeywell Safety Products USA, Inc. (079287321) Establishment Name Address ID/FEI Business Operations James Alexander 040756421 manufacture(0498-3334) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc 079287321 pack(0498-4293) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0203, 0498-0750, 0498-0800, 0498-0801) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0143) Establishment Name Address ID/FEI Business Operations Sion Medical Biotext 532775194 manufacture(0498-0121) Establishment Name Address ID/FEI Business Operations Safetec of America Inc 874965262 manufacture(0498-0733)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.