Felbamate by Alvogen Inc. FELBAMATE tablet
Felbamate by
Drug Labeling and Warnings
Felbamate by is a Prescription medication manufactured, distributed, or labeled by Alvogen Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Before Prescribing felbamate tablets, the physician should be thoroughly familiar with the details of this prescribing information.
FELBAMATE TABLETS SHOULD NOT BE USED BY PATIENTS UNTIL THERE HAS BEEN A COMPLETE DISCUSSION OF THE RISKS AND THE PATIENT, PARENT OR GUARDIAN HAS BEEN PROVIDED THE FELBAMATE TABLETS WRITTEN ACKNOWLEDGMENT (SEE PATIENT/PHYSICIAN ACKNOWLEDGMENT FORM).
-
BOXED WARNING
(What is this?)
WARNING
1. APLASTIC ANEMIA
THE USE OF FELBAMATE TABLETS IS ASSOCIATED WITH A MARKED INCREASE IN THE INCIDENCE OF APLASTIC ANEMIA. ACCORDINGLY, FELBAMATE TABLETS SHOULD ONLY BE USED IN PATIENTS WHOSE EPILEPSY IS SO SEVERE THAT THE RISK OF APLASTIC ANEMIA IS DEEMED ACCEPTABLE IN LIGHT OF THE BENEFITS CONFERRED BY ITS USE (SEE INDICATIONS). ORDINARILY, A PATIENT SHOULD NOT BE PLACED ON AND/OR CONTINUED ON FELBAMATE TABLETS WITHOUT CONSIDERATION OF APPROPRIATE EXPERT HEMATOLOGIC CONSULTATION.
AMONG FELBAMATE TABLET TREATED PATIENTS, APLASTIC ANEMIA (PANCYTOPENIA IN THE PRESENCE OF A BONE MARROW LARGELY DEPLETED OF HEMATOPOIETIC PRECURSORS) OCCURS AT AN INCIDENCE THAT MAY BE MORE THAN A 100-FOLD GREATER THAN THAT SEEN IN THE UNTREATED POPULATION (I.E., 2 TO 5 PER MILLION PERSONS PER YEAR). THE RISK OF DEATH IN PATIENTS WITH APLASTIC ANEMIA GENERALLY VARIES AS A FUNCTION OF ITS SEVERITY AND ETIOLOGY; CURRENT ESTIMATES OF THE OVERALL CASE FATALITY RATE ARE IN THE RANGE OF 20% TO 30%, BUT RATES AS HIGH AS 70% HAVE BEEN REPORTED IN THE PAST.
THERE ARE TOO FEW FELBAMATE TABLET ASSOCIATED CASES, AND TOO LITTLE KNOWN ABOUT THEM TO PROVIDE A RELIABLE ESTIMATE OF THE SYNDROME'S INCIDENCE OR ITS CASE FATALITY RATE OR TO IDENTIFY THE FACTORS, IF ANY, THAT MIGHT CONCEIVABLY BE USED TO PREDICT WHO IS AT GREATER OR LESSER RISK.
IN MANAGING PATIENTS ON FELBAMATE TABLETS, IT SHOULD BE BORNE IN MIND THAT THE CLINICAL MANIFESTATION OF APLASTIC ANEMIA MAY NOT BE SEEN UNTIL AFTER A PATIENT HAS BEEN ON FELBAMATE TABLETS FOR SEVERAL MONTHS (E.G., ONSET OF APLASTIC ANEMIA AMONG FELBAMATE TABLET EXPOSED PATIENTS FOR WHOM DATA ARE AVAILABLE HAS RANGED FROM 5 TO 30 WEEKS). HOWEVER, THE INJURY TO BONE MARROW STEM CELLS THAT IS HELD TO BE ULTIMATELY RESPONSIBLE FOR THE ANEMIA MAY OCCUR WEEKS TO MONTHS EARLIER. ACCORDINGLY, PATIENTS WHO ARE DISCONTINUED FROM FELBAMATE TABLETS REMAIN AT RISK FOR DEVELOPING ANEMIA FOR A VARIABLE, AND UNKNOWN, PERIOD AFTERWARDS.
IT IS NOT KNOWN WHETHER OR NOT THE RISK OF DEVELOPING APLASTIC ANEMIA CHANGES WITH DURATION OF EXPOSURE. CONSEQUENTLY, IT IS NOT SAFE TO ASSUME THAT A PATIENT WHO HAS BEEN ON FELBAMATE TABLETS WITHOUT SIGNS OF HEMATOLOGIC ABNORMALITY FOR LONG PERIODS OF TIME IS WITHOUT RISK.
IT IS NOT KNOWN WHETHER OR NOT THE DOSE OF FELBAMATE TABLETS AFFECTS THE INCIDENCE OF APLASTIC ANEMIA.
IT IS NOT KNOWN WHETHER OR NOT CONCOMITANT USE OF ANTIEPILEPTIC DRUGS AND/OR OTHER DRUGS AFFECTS THE INCIDENCE OF APLASTIC ANEMIA.
APLASTIC ANEMIA TYPICALLY DEVELOPS WITHOUT PREMONITORY CLINICAL OR LABORATORY SIGNS, THE FULL BLOWN SYNDROME PRESENTING WITH SIGNS OF INFECTION, BLEEDING, OR ANEMIA. ACCORDINGLY, ROUTINE BLOOD TESTING CANNOT BE RELIABLY USED TO REDUCE THE INCIDENCE OF APLASTIC ANEMIA, BUT, IT WILL, IN SOME CASES, ALLOW THE DETECTION OF THE HEMATOLOGIC CHANGES BEFORE THE SYNDROME DECLARES ITSELF CLINICALLY. FELBAMATE TABLETS SHOULD BE DISCONTINUED IF ANY EVIDENCE OF BONE MARROW DEPRESSION OCCURS.
2. HEPATIC FAILURE
EVALUATION OF POSTMARKETING EXPERIENCE SUGGESTS THAT ACUTE LIVER FAILURE IS ASSOCIATED WITH THE USE OF FELBAMATE TABLETS. THE REPORTED RATE IN THE U.S. HAS BEEN ABOUT SIX CASES OF LIVER FAILURE LEADING TO DEATH OR TRANSPLANT PER 75,000 PATIENT YEARS OF USE. THIS RATE IS AN UNDERESTIMATE BECAUSE OF UNDER REPORTING, AND THE TRUE RATE COULD BE CONSIDERABLY GREATER THAN THIS. FOR EXAMPLE, IF THE REPORTING RATE IS 10%, THE TRUE RATE WOULD BE ONE CASE PER 1,250 PATIENT YEARS OF USE.
OF THE CASES REPORTED, ABOUT 67% RESULTED IN DEATH OR LIVER TRANSPLANTATION, USUALLY WITHIN 5 WEEKS OF THE ONSET OF SIGNS AND SYMPTOMS OF LIVER FAILURE. THE EARLIEST ONSET OF SEVERE HEPATIC DYSFUNCTION FOLLOWED SUBSEQUENTLY BY LIVER FAILURE WAS 3 WEEKS AFTER INITIATION OF FELBAMATE TABLETS. ALTHOUGH SOME REPORTS DESCRIBED DARK URINE AND NONSPECIFIC PRODROMAL SYMPTOMS (E.G., ANOREXIA, MALAISE, AND GASTROINTESTINAL SYMPTOMS), IN OTHER REPORTS IT WAS NOT CLEAR IF ANY PRODROMAL SYMPTOMS PRECEDED THE ONSET OF JAUNDICE.
IT IS NOT KNOWN WHETHER OR NOT THE RISK OF DEVELOPING HEPATIC FAILURE CHANGES WITH DURATION OF EXPOSURE.
IT IS NOT KNOWN WHETHER OR NOT THE DOSAGE OF FELBAMATE TABLETS AFFECTS THE INCIDENCE OF HEPATIC FAILURE.
IT IS NOT KNOWN WHETHER CONCOMITANT USE OF OTHER ANTIEPILEPTIC DRUGS AND/OR OTHER DRUGS AFFECT THE INCIDENCE OF HEPATIC FAILURE.
FELBAMATE TABLETS SHOULD NOT BE PRESCRIBED FOR ANYONE WITH A HISTORY OF HEPATIC DYSFUNCTION.
TREATMENT WITH FELBAMATE TABLETS SHOULD BE INITIATED ONLY IN INDIVIDUALS WITHOUT ACTIVE LIVER DISEASE AND WITH NORMAL BASELINE SERUM TRANSAMINASES. IT HAS NOT BEEN PROVED THAT PERIODIC SERUM TRANSAMINASE TESTING WILL PREVENT SERIOUS INJURY BUT IT IS GENERALLY BELIEVED THAT EARLY DETECTION OF DRUG-INDUCED HEPATIC INJURY ALONG WITH IMMEDIATE WITHDRAWAL OF THE SUSPECT DRUG ENHANCES THE LIKELIHOOD FOR RECOVERY. THERE IS NO INFORMATION AVAILABLE THAT DOCUMENTS HOW RAPIDLY PATIENTS CAN PROGRESS FROM NORMAL LIVER FUNCTION TO LIVER FAILURE, BUT OTHER DRUGS KNOWN TO BE HEPATOTOXINS CAN CAUSE LIVER FAILURE RAPIDLY (E.G., FROM NORMAL ENZYMES TO LIVER FAILURE IN 2 TO 4 WEEKS). ACCORDINGLY, MONITORING OF SERUM TRANSAMINASE LEVELS (AST AND ALT) IS RECOMMENDED AT BASELINE AND PERIODICALLY THEREAFTER. WHILE THE MORE FREQUENT THE MONITORING THE GREATER THE CHANCES OF EARLY DETECTION, THE PRECISE SCHEDULE FOR MONITORING IS A MATTER OF CLINICAL JUDGEMENT.
FELBAMATE TABLETS SHOULD BE DISCONTINUED IF EITHER SERUM AST OR SERUM ALT LEVELS BECOME INCREASED ≥ 2 TIMES THE UPPER LIMIT OF NORMAL, OR IF CLINICAL SIGNS AND SYMPTOMS SUGGEST LIVER FAILURE (SEE PRECAUTIONS). PATIENTS WHO DEVELOP EVIDENCE OF HEPATOCELLULAR INJURY WHILE ON FELBAMATE TABLETS AND ARE WITHDRAWN FROM THE DRUG FOR ANY REASON SHOULD BE PRESUMED TO BE AT INCREASED RISK FOR LIVER INJURY IF FELBAMATE TABLETS ARE REINTRODUCED. ACCORDINGLY, SUCH PATIENTS SHOULD NOT BE CONSIDERED FOR RE-TREATMENT.
-
DESCRIPTION
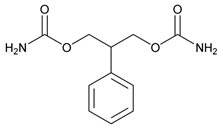
Felbamate is an antiepileptic available as 400 mg and 600 mg tablets for oral administration. Its chemical name is 2-Phenyl-1,3-propanediol dicarbamate.
Felbamate, USP is a white to off-white powder with a characteristic odor. It is very slightly soluble in water, slightly soluble in ethanol, sparingly soluble in methanol, and freely soluble in dimethyl sulfoxide. The molecular weight is 238.24; felbamate's molecular formula is C11H14N2O4; its structural formula is:
The inactive ingredients for felbamate tablets, USP 400 mg and 600 mg are colloidal silicon dioxide, corn starch, croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
-
CLINICAL PHARMACOLOGY
Mechanism of Action:
The mechanism by which felbamate exerts its anticonvulsant activity is unknown, but in animal test systems designed to detect anticonvulsant activity, felbamate has properties in common with other marketed anticonvulsants. Felbamate is effective in mice and rats in the maximal electroshock test, the subcutaneous pentylenetetrazol seizure test, and the subcutaneous picrotoxin seizure test. Felbamate also exhibits anticonvulsant activity against seizures induced by intracerebroventricular administration of glutamate in rats and N-methyl-D,L-aspartic acid in mice. Protection against maximal electroshock-induced seizures suggests that felbamate may reduce seizure spread, an effect possibly predictive of efficacy in generalized tonic-clonic or partial seizures. Protection against pentylenetetrazol-induced seizures suggests that felbamate may increase seizure threshold, an effect considered to be predictive of potential efficacy in absence seizures.
Receptor-binding studies in vitro indicate that felbamate has weak inhibitory effects on GABA-receptor binding, benzodiazepine receptor binding, and is devoid of activity at the MK-801 receptor binding site of the NMDA receptor-ionophore complex. However, felbamate does interact as an antagonist at the strychnine-insensitive glycine recognition site of the NMDA receptor-ionophore complex. Felbamate is not effective in protecting chick embryo retina tissue against the neurotoxic effects of the excitatory amino acid agonists NMDA, kainite, or quisqualate in vitro.
The monocarbamate, p-hydroxy, and 2-hydroxy metabolites were inactive in the maximal electroshock-induced seizure test in mice. The monocarbamate and p-hydroxy metabolites had only weak (0.2 to 0.6) activity compared with felbamate in the subcutaneous pentylenetetrazol seizure test. These metabolites did not contribute significantly to the anticonvulsant action of felbamate.
Pharmacokinetics:
The numbers in the pharmacokinetic section are mean ± standard deviation.
Felbamate is well-absorbed after oral administration. Over 90% of the radioactivity after a dose of 1000 mg 14C felbamate was found in the urine. Absolute bioavailability (oral vs. parenteral) has not been measured. The tablet and suspension were each shown to be bioequivalent to the capsule used in clinical trials, and pharmacokinetic parameters of the tablet and suspension are similar. There was no effect of food on absorption of the tablet; the effect of food on absorption of the suspension has not been evaluated.
Following oral administration, felbamate is the predominant plasma species (about 90% of plasma radioactivity). About 40% to 50% of absorbed dose appears unchanged in urine, and an additional 40% is present as unidentified metabolites and conjugates. About 15% is present as parahydroxyfelbamate, 2-hydroxyfelbamate, and felbamate monocarbamate, none of which have significant anticonvulsant activity.
Binding of felbamate to human plasma protein was independent of felbamate concentrations between 10 micrograms/mL and 310 micrograms/mL. Binding ranged from 22% to 25%, mostly to albumin, and was dependent on the albumin concentration.
Felbamate is excreted with a terminal half-life of 20 to 23 hours, which is unaltered after multiple doses. Clearance after a single 1200 mg dose is 26 ± 3 mL/hr/kg, and after multiple daily doses of 3600 mg is 30 ± 8 mL/hr/kg. The apparent volume of distribution was 756 ± 82 mL/kg after a 1200 mg dose. Felbamate Cmax and AUC are proportionate to dose after single and multiple doses over a range of 100 mg to 800 mg single doses and 1200 mg to 3600 mg daily doses. Cmin (trough) blood levels are also dose proportional. Multiple daily doses of 1200 mg, 2400 mg, and 3600 mg gave Cmin values of 30 ± 5, 55 ± 8, and 83 ± 21 micrograms/mL (N = 10 patients). Linear and dose proportional pharmacokinetics were also observed at doses above 3600 mg/day up to the maximum dose studied of 6000 mg/day. Felbamate gave dose proportional steady-state peak plasma concentrations in children age 4 to 12 over a range of 15 mg/kg/day, 30 mg/kg/day, and 45 mg/kg/day with peak concentrations of 17 micrograms/mL, 32 micrograms/mL, and 49 micrograms/mL.
The effects of race and gender on felbamate pharmacokinetics have not been systematically evaluated, but plasma concentrations in males (N = 5) and females (N = 4) given felbamate have been similar. The effects of felbamate kinetics on hepatic functional impairment have not been evaluated.
Renal Impairment:
Felbamate's single dose monotherapy pharmacokinetic parameters were evaluated in 12 otherwise healthy individuals with renal impairment. There was a 40% to 50% reduction in total body clearance and 9 to 15 hours prolongation of half-life in renally impaired subjects compared to that in subjects with normal renal function. Reduced felbamate clearance and a longer half-life were associated with diminishing renal function.
Pharmacodynamics:
Typical Physiologic Responses:
1. Cardiovascular: In adults, there is no effect of felbamate on blood pressure. Small but statistically significant mean increases in heart rate were seen during adjunctive therapy and monotherapy; however, these mean increases of up to 5 bpm were not clinically significant. In children, no clinically relevant changes in blood pressure or heart rate were seen during adjunctive therapy or monotherapy with felbamate.
2. Other Physiologic Effects: The only other change in vital signs was a mean decrease of approximately one respiration per minute in respiratory rate during adjunctive therapy in children. In adults, statistically significant mean reductions in body weight were observed during felbamate monotherapy and adjunctive therapy. In children, there were mean decreases in body weight during adjunctive therapy and monotherapy; however, these mean changes were not statistically significant. These mean reductions in adults and children were approximately 5% of the mean weights at baseline.
-
CLINICAL STUDIES
The results of controlled clinical trials established the efficacy of felbamate as monotherapy and adjunctive therapy in adults with partial-onset seizures with or without secondary generalization and in partial and generalized seizures associated with Lennox-Gastaut syndrome in children.
Felbamate Monotherapy Trials in Adults
Felbamate (3600 mg/day given QID) and low-dose valproate (15 mg/kg/day) were compared as monotherapy during a 112-day treatment period in a multicenter and a single-center double-blind efficacy trial. Both trials were conducted according to an identical study design. During a 56-day baseline period, all patients had at least four partial-onset seizures per 28 days and were receiving one antiepileptic drug at a therapeutic level, the most common being carbamazepine. In the multicenter trial, baseline seizure frequencies were 12.4 per 28 days in the felbamate group and 21.3 per 28 days in the low-dose valproate group. In the single-center trial, baseline seizure frequencies were 18.1 per 28 days in the felbamate group and 15.9 per 28 days in the low-dose valproate group. Patients were converted to monotherapy with felbamate or low-dose valproic acid during the first 28 days of the 112-day treatment period. Study endpoints were completion of 112 study days or fulfilling an escape criterion. Criteria for escape relative to baseline were: (1) 2-fold increase in monthly seizure frequency, (2) 2-fold increase in highest 2-day seizure frequency, (3) single generalized tonic-clonic seizure (GTC) if none occurred during baseline, or (4) significant prolongation of GTCs. The primary efficacy variable was the number of patients in each treatment group who met escape criteria.
In the multicenter trial, the percentage of patients who met escape criteria was 40% (18/45) in the felbamate group and 78% (39/50) in the low-dose valproate group. In the single-center trial, the percentage of patients who met escape criteria was 14% (3/21) in the felbamate group and 90% (19/21) in the low-dose valproate group. In both trials, the difference in the percentage of patients meeting escape criteria was statistically significant (P < 0.001) in favor of felbamate. These two studies by design were intended to demonstrate the effectiveness of felbamate monotherapy. The studies were not designed or intended to demonstrate comparative efficacy of the two drugs. For example, valproate was not used at the maximally effective dose.
Felbamate Adjunctive Therapy Trials in Adults
A double-blind, placebo-controlled crossover trial consisted of two 10-week outpatient treatment periods. Patients with refractory partial-onset seizures who were receiving phenytoin and carbamazepine at therapeutic levels were administered felbamate as add-on therapy at a starting dosage of 1400 mg/day in three divided doses, which was increased to 2600 mg/day in three divided doses. Among the 56 patients who completed the study, the baseline seizure frequency was 20 per month. Patients treated with felbamate had fewer seizures than patients treated with placebo for each treatment sequence. There was a 23% (P = 0.018) difference in percentage seizure frequency reduction in favor of felbamate.
Felbamate 3600 mg/day given QID and placebo were compared in a 28-day double-blind add-on trial in patients who had their standard antiepileptic drugs reduced while undergoing evaluations for surgery of intractable epilepsy. All patients had confirmed partial-onset seizures with or without generalization, seizure frequency during surgical evaluation not exceeding an average of four partial seizures per day or more than one generalized seizure per day, and a minimum average of one partial or generalized tonic-clonic seizure per day for the last 3 days of the surgical evaluation. The primary efficacy variable was time to fourth seizure after randomization to treatment with felbamate or placebo. Thirteen (46%) of 28 patients in the felbamate group versus 29 (88%) of 33 patients in the placebo group experienced a fourth seizure. The median times to fourth seizure were greater than 28 days in the felbamate group and 5 days in the placebo group. The difference between felbamate and placebo in time to fourth seizure was statistically significant (P = 0.002) in favor of felbamate.
Felbamate Adjunctive Therapy Trial in Children with Lennox-Gastaut Syndrome
In a 70-day double-blind, placebo-controlled add-on trial in the Lennox-Gastaut syndrome, felbamate 45 mg/kg/day given QID was superior to placebo in controlling the multiple seizure types associated with this condition. Patients had at least 90 atonic and/or atypical absence seizures per month while receiving therapeutic dosages of one or two other antiepileptic drugs. Patients had a past history of using an average of eight antiepileptic drugs. The most commonly used antiepileptic drug during the baseline period was valproic acid. The frequency of all types of seizures during the baseline period was 1,617 per month in the felbamate group and 716 per month in the placebo group. Statistically significant differences in the effect on seizure frequency favored felbamate over placebo for total seizures (26% reduction vs. 5% increase, P < 0.001), atonic seizures (44% reduction vs. 7% reduction, P = 0.002), and generalized tonic-clonic seizures (40% reduction vs. 12% increase, P = 0.017). Parent/guardian global evaluations based on impressions of quality of life with respect to alertness, verbal responsiveness, general well-being, and seizure control significantly (P < 0.001) favored felbamate over placebo.
When efficacy was analyzed by gender in four well-controlled trials of felbamate as adjunctive and monotherapy for partial-onset seizures and Lennox-Gastaut syndrome, a similar response was seen in 122 males and 142 females.
-
INDICATIONS AND USAGE
Felbamate tablets, USP are not indicated as a first line antiepileptic treatment (see WARNINGS). Felbamate tablets are recommended for use only in those patients who respond inadequately to alternative treatments and whose epilepsy is so severe that a substantial risk of aplastic anemia and/or liver failure is deemed acceptable in light of the benefits conferred by its use.
If these criteria are met and the patient has been fully advised of the risk, and has provided written acknowledgment, Felbamate tablets can be considered for either monotherapy or adjunctive therapy in the treatment of partial seizures, with and without generalization, in adults with epilepsy and as adjunctive therapy in the treatment of partial and generalized seizures associated with Lennox-Gastaut syndrome in children.
- CONTRAINDICATIONS
-
WARNINGS
See Boxed Warning regarding aplastic anemia and hepatic failure. Antiepileptic drugs should not be suddenly discontinued because of the possibility of increasing seizure frequency.
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs) including felbamate, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis Indication
Placebo Patients with Events Per 1,000 Patients
Drug Patients with Events Per 1,000 Patients
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients
Risk Difference: Additional Drug Patients with Events Per 1,000 Patients
Epilepsy
1.0
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1.0
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing felbamate or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
-
PRECAUTIONS
Dosage Adjustment in the Renally Impaired:
A study in otherwise healthy individuals with renal dysfunction indicated that prolonged half-life and reduced clearance of felbamate are associated with diminishing renal function. Felbamate should be used with caution in patients with renal dysfunction (see DOSAGE AND ADMINISTRATION).
Information for Patients:
Patients should be informed that the use of felbamate is associated with aplastic anemia and hepatic failure, potentially fatal conditions acutely or over a long term.
The physician should obtain written acknowledgment prior to initiation of felbamate therapy (see PATIENT/PHYSICIAN ACKNOWLEDGMENT FORM section).
Patients should be instructed to read the Medication Guide supplied as required by law when felbamate is dispensed. The complete text of the Medication Guide is reprinted at the end of this document.
Aplastic anemia in the general population is relatively rare. The absolute risk for the individual patient is not known with any degree of reliability, but patients on felbamate may be at more than a 100-fold greater risk for developing the syndrome than the general population.
The long term outlook for patients with aplastic anemia is variable. Although many patients are apparently cured, others require repeated transfusions and other treatments for relapses, and some, although surviving for years, ultimately develop serious complications that sometimes prove fatal (e.g., leukemia).
At present there is no way to predict who is likely to get aplastic anemia, nor is there a documented effective means to monitor the patient so as to avoid and/or reduce the risk. Patients with a history of any blood dyscrasia should not receive felbamate.
Patients should be advised to be alert for signs of infection, bleeding, easy bruising, or signs of anemia (fatigue, weakness, lassitude, etc.) and should be advised to report to the physician immediately if any such signs or symptoms appear.
Hepatic failure in the general population is relatively rare. The absolute risk for an individual patient is not known with any degree of reliability but patients on felbamate are at a greater risk for developing hepatic failure than the general population.
At present, there is no way to predict who is likely to develop hepatic failure, however, patients with a history of hepatic dysfunction should not be started on felbamate.
Patients should be advised to follow their physician's directives for liver function testing both before starting felbamate and at frequent intervals while taking felbamate.
Patients should be advised to be alert for signs of liver dysfunction (jaundice, anorexia, gastrointestinal complaints, malaise, etc.) and to report them to their doctor immediately if they should occur.
Laboratory Tests:
Full hematologic evaluations should be performed before felbamate therapy, frequently during therapy, and for a significant period of time after discontinuation of felbamate therapy. While it might appear prudent to perform frequent CBCs in patients continuing on felbamate, there is no evidence that such monitoring will allow early detection of marrow suppression before aplastic anemia occurs. (See BOXED WARNINGS). Complete pretreatment blood counts, including platelets and reticulocytes should be obtained as a baseline. If any hematologic abnormalities are detected during the course of treatment, immediate consultation with a hematologist is advised. Felbamate should be discontinued if any evidence of bone marrow depression occurs.
See Box Warnings for recommended monitoring of serum transaminases. If significant, confirmed liver abnormalities are detected during the course of felbamate treatment, felbamate should be discontinued immediately with continued liver function monitoring until values return to normal. (See PATIENT/PHYSICIAN ACKNOWLEDGMENT FORM.)
Suicidal Thinking and Behavior:
Patients, their caregivers, and families should be counseled that AEDs, including felbamate, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Pregnancy:
Patients should be encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 (see PREGNANCY section).
Drug Interactions:
The drug interaction data described in this section were obtained from controlled clinical trials and studies involving otherwise healthy adults with epilepsy.
Use in Conjunction with Other Antiepileptic Drugs
(See DOSAGE AND ADMINISTRATION). The addition of felbamate to antiepileptic drugs (AEDs) affects the steady-state plasma concentrations of AEDs. The net effect of these interactions is summarized in Table 2:
Table 2. Steady-State Plasma Concentrations of Felbamate When Coadministered With Other AEDs - * No significant effect.
- † Not administered but an active metabolite of carbamazepine.
AED Coadministered
AED Concentration
Felbamate Concentration
Phenytoin
↑
↓
Valproate
↑
↔*
Carbamazepine (CBZ)
†CBZ epoxide↓
↑
↓
Phenobarbital
↑
↓
Specific Effects of Felbamate on Other Antiepileptic Drugs:
Phenytoin:
Felbamate causes an increase in steady-state phenytoin plasma concentrations. In ten otherwise healthy subjects with epilepsy ingesting phenytoin, the steady-state trough (Cmin) phenytoin plasma concentration was 17 ± 5 micrograms/mL. The steady-state Cmin increased to 21 ± 5 micrograms/mL when 1200 mg/day of felbamate was coadministered. Increasing the felbamate dose to 1800 mg/day in six of these subjects increased the steady-state phenytoin Cmin to 25 ± 7 micrograms/mL. In order to maintain phenytoin levels, limit adverse experiences, and achieve the felbamate dose of 3600 mg/day, a phenytoin dose reduction of approximately 40% was necessary for eight of these ten subjects.
In a controlled clinical trial, a 20% reduction of the phenytoin dose at the initiation of felbamate therapy resulted in phenytoin levels comparable to those prior to felbamate administration.
Carbamazepine:
Felbamate causes a decrease in the steady-state carbamazepine plasma concentrations and an increase in the steady-state carbamazepine epoxide plasma concentration. In nine otherwise healthy subjects with epilepsy ingesting carbamazepine, the steady-state trough (Cmin) carbamazepine concentration was 8 ± 2 micrograms/mL. The carbamazepine steady-state Cmin decreased 31% to 5 ± 1 micrograms/mL when felbamate (3000 mg/day, divided into three doses) was coadministered. Carbamazepine epoxide steady-state Cmin concentrations increased 57% from 1 ± 0.3 to 1.6 ± 0.4 micrograms/mL with the addition of felbamate.
In clinical trials, similar changes in carbamazepine and carbamazepine epoxide were seen.
Valproate:
Felbamate causes an increase in steady-state valproate concentrations. In four subjects with epilepsy ingesting valproate, the steady-state trough (Cmin) valproate plasma concentration was 63 ± 16 micrograms/mL. The steady-state Cmin increased to 78 ± 14 micrograms/mL when 1200 mg/day of felbamate was coadministered. Increasing the felbamate dose to 2400 mg/day increased the steady-state valproate Cmin to 96 ± 25 micrograms/mL. Corresponding values for free valproate Cmin concentrations were 7 ± 3, 9 ± 4, and 11 ± 6 micrograms/mL for 0, 1200, and 2400 mg/day felbamate, respectively. The ratios of the AUCs of unbound valproate to the AUCs of the total valproate were 11.1%, 13%, and 11.5%, with coadministration of 0, 1200, and 2400 mg/day of felbamate, respectively. This indicates that the protein binding of valproate did not change appreciably with increasing doses of felbamate.
Phenobarbital:
Coadministration of felbamate with phenobarbital causes an increase in phenobarbital plasma concentrations. In 12 otherwise healthy male volunteers ingesting phenobarbital, the steady-state trough (Cmin) phenobarbital concentration was 14.2 micrograms/mL. The steady-state Cmin concentration increased to 17.8 micrograms/mL when 2400 mg/day of felbamate was coadministered for one week.
Effects of Other Antiepileptic Drugs on Felbamate:
Phenytoin:
Phenytoin causes an approximate doubling of the clearance of felbamate at steady-state and, therefore, the addition of phenytoin causes an approximate 45% decrease in the steady-state trough concentrations of felbamate as compared to the same dose of felbamate given as monotherapy.
Carbamazepine:
Carbamazepine causes an approximate 50% increase in the clearance of felbamate at steady-state and, therefore, the addition of carbamazepine results in an approximate 40% decrease in the steady-state trough concentrations of felbamate as compared to the same dose of felbamate given as monotherapy.
Effects of Antacids on Felbamate:
The rate and extent of absorption of a 2400 mg dose of felbamate as monotherapy given as tablets was not affected when coadministered with antacids.
Effects of Erythromycin on Felbamate:
The coadministration of erythromycin (1000 mg/day) for 10 days did not alter the pharmacokinetic parameters of Cmax, Cmin, AUC, Cl/kg or Tmax at felbamate daily doses of 3000 mg/day or 3600 mg/day in 10 otherwise healthy subjects with epilepsy.
Effects of Felbamate on Low-Dose Combination Oral Contraceptives:
A group of 24 nonsmoking, healthy white female volunteers established on an oral contraceptive regimen containing 30 mcg ethinyl estradiol and 75 mcg gestodene for at least 3 months received 2400 mg/day of felbamate from midcycle (day 15) to midcycle (day 14) of two consecutive oral contraceptive cycles. Felbamate treatment resulted in a 42% decrease in the gestodene AUC0-24, but no clinically relevant effect was observed on the pharmacokinetic parameters of ethinyl estradiol. No volunteer showed hormonal evidence of ovulation, but one volunteer reported intermenstrual bleeding during felbamate treatment.
Drug/Laboratory Test Interactions:
There are no known interactions of felbamate with commonly used laboratory tests.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Carcinogenicity studies were conducted in mice and rats. Mice received felbamate as a feed admixture for 92 weeks at doses of 300 mg/kg, 600 mg/kg, and 1200 mg/kg and rats were also dosed by feed admixture for 104 weeks at doses of 30 mg/kg, 100 mg/kg, and 300 (males) mg/kg or 10 mg/kg, 30 mg/kg, and 100 (females) mg/kg. The maximum doses in these studies produced steady-state plasma concentrations that were equal to or less than the steady-state plasma concentrations in epileptic patients receiving 3600 mg/day. There was a statistically significant increase in hepatic cell adenomas in high-dose male and female mice and in high-dose female rats. Hepatic hypertrophy was significantly increased in a dose-related manner in mice, primarily males, but also in females. Hepatic hypertrophy was not found in female rats. The relationship between the occurrence of benign hepatocellular adenomas and the finding of liver hypertrophy resulting from liver enzyme induction has not been examined. There was a statistically significant increase in benign interstitial cell tumors of the testes in high-dose male rats receiving felbamate. The relevance of these findings to humans is unknown.
As a result of the synthesis process, felbamate could contain small amounts of two known animal carcinogens, the genotoxic compound ethyl carbamate (urethane) and the nongenotoxic compound methyl carbamate. It is theoretically possible that a 50 kg patient receiving 3600 mg of felbamate could be exposed to up to 0.72 micrograms of urethane and 1800 micrograms of methyl carbamate. These daily doses are approximately 1/35,000 (urethane) and 1/5,500 (methyl carbamate) on a mg/kg basis, and 1/10,000 (urethane) and 1/1,600 (methyl carbamate) on a mg/m2 basis, of the dose levels shown to be carcinogenic in rodents. Any presence of these two compounds in felbamate used in the lifetime carcinogenicity studies was inadequate to cause tumors.
Microbial and mammalian cell assays revealed no evidence of mutagenesis in the Ames Salmonella/microsome plate test, CHO/HGPRT mammalian cell forward gene mutation assay, sister chromatid exchange assay in CHO cells, and bone marrow cytogenetics assay.
Reproduction and fertility studies in rats showed no effects on male or female fertility at oral doses of up to 13.9 times the human total daily dose of 3600 mg on a mg/kg basis, or up to 3 times the human total daily dose on a mg/m2 basis.
Pregnancy
Pregnancy: Pregnancy Category C
The incidence of malformations was not increased compared to control in offspring of rats or rabbits given doses up to 13.9 times (rat) and 4.2 times (rabbit) the human daily dose on a mg/kg basis, or 3 times (rat) and less than 2 times (rabbit) the human daily dose on a mg/m2 basis. However, in rats, there was a decrease in pup weight and an increase in pup deaths during lactation. The cause for these deaths is not known. The no effect dose for rat pup mortality was 6.9 times the human dose on a mg/kg basis or 1.5 times the human dose on a mg/m2 basis.
Placental transfer of felbamate occurs in rat pups. There are, however, no studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
To provide information regarding the effects of in utero exposure to felbamate, physicians are advised to recommend that pregnant patients taking felbamate enroll in the NAAED Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Nursing Mothers:
Felbamate has been detected in human milk. The effect on the nursing infant is unknown (see PREGNANCY section).
Pediatric Use:
The safety and effectiveness of felbamate in children other than those with Lennox-Gastaut syndrome has not been established.
Geriatric Use:
No systematic studies in geriatric patients have been conducted. Clinical studies of felbamate did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dosage selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Alvogen, Inc. at 1-866-770-3024 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
The most common adverse reactions seen in association with felbamate in adults during monotherapy are anorexia, vomiting, insomnia, nausea, and headache. The most common adverse reactions seen in association with felbamate in adults during adjunctive therapy are anorexia, vomiting, insomnia, nausea, dizziness, somnolence, and headache.
The most common adverse reactions seen in association with felbamate in children during adjunctive therapy are anorexia, vomiting, insomnia, headache, and somnolence.
The dropout rate because of adverse experiences or intercurrent illnesses among adult felbamate patients was 12 percent (120/977). The dropout rate because of adverse experiences or intercurrent illnesses among pediatric felbamate patients was 6% (22/357). In adults, the body systems associated with causing these withdrawals in order of frequency were: digestive (4.3%), psychological (2.2%), whole body (1.7%), neurological (1.5%) and dermatological (1.5%). In children, the body systems associated with causing these withdrawals in order of frequency were: digestive (1.7%), neurological (1.4%), dermatological (1.4%), psychological (1.1%), and whole body (1%). In adults, specific events with an incidence of 1% or greater associated with causing these withdrawals, in order of frequency were: anorexia (1.6%), nausea (1.4%), rash (1.2%), and weight decrease (1.1%). In children, specific events with an incidence of 1% or greater associated with causing these withdrawals, in order of frequency was rash (1.1%).
Incidence in Clinical Trials:
The prescriber should be aware that the figures cited in the following table cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different investigators, treatments, and uses including the use of felbamate as adjunctive therapy where the incidence of adverse events may be higher due to drug interactions. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence rate in the population studied.
Adults
Incidence in Controlled Clinical Trials--Monotherapy Studies in Adults:
The table that follows enumerates adverse events that occurred at an incidence of 2% or more among 58 adult patients who received felbamate monotherapy at dosages of 3600 mg/day in double-blind controlled trials. Table 3 presents reported adverse events that were classified using standard WHO-based dictionary terminology.
Table 3. Adults Treatment-Emergent Adverse Event Incidence in Controlled Monotherapy Trials - * 3600 mg/day
- † 15 mg/kg/day
Felbamate*
(N = 58)
Low Dose Valproate†
(N = 50)
Body System Event
%
%
Body as a Whole
Fatigue
Weight Decrease
Face Edema
6.9
3.4
3.4
4.0
0
0
Central Nervous System
Insomnia
Headache
Anxiety
8.6
6.9
5.2
4.0
18
2
Dermatological
Acne
Rash
3.4
3.4
0
0
Digestive
Dyspepsia
Vomiting
Constipation
Diarrhea
SGPT Increased
8.6
8.6
6.9
5.2
5.2
2.0
2.0
2.0
0
2.0
Metabolic/Nutritional
Hypophosphatemia
3.4
0
Respiratory
Upper Respiratory Tract Infection
Rhinitis
8.6
6.9
4.0
0
Special Senses
Diplopia
Otitis Media
3.4
3.4
4.0
0
Urogenital
Intramenstrual Bleeding
Urinary Tract Infection
3.4
3.4
0
2.0
Incidence in Controlled Add-On Clinical Studies in Adults:
Table 4 enumerates adverse events that occurred at an incidence of 2% or more among 114 adult patients who received felbamate adjunctive therapy in add-on controlled trials at dosages up to 3600 mg/day. Reported adverse events were classified using standard WHO-based dictionary terminology.
Many adverse experiences that occurred during adjunctive therapy may be a result of drug interactions. Adverse experiences during adjunctive therapy typically resolved with conversion to monotherapy, or with adjustment of the dosage of other antiepileptic drugs.
Table 4. Adults Treatment-Emergent Adverse Event Incidence in Controlled Add-On Trials Felbamate
(N = 114)
Placebo
(N = 43)
Body System/Event
%
%
Body as a Whole
Fatigue
Fever
Chest Pain
16.8
2.6
2.6
7.0
4.7
0
Central Nervous System
Headache
Somnolence
Dizziness
Insomnia
Nervousness
Tremor
Anxiety
Gait Abnormal
Depression
Paraesthesia
Ataxia
Mouth Dry
Stupor
36.8
19.3
18.4
17.5
7.0
6.1
5.3
5.3
5.3
3.5
3.5
2.6
2.6
9.3
7.0
14
7.0
2.3
2.3
4.7
0
0
2.3
0
0
0
Dermatological
Rash
3.5
4.7
Digestive
Nausea
Anorexia
Vomiting
Dyspepsia
Constipation
Diarrhea
Abdominal Pain
SGPT Increased
34.2
19.3
16.7
12.3
11.4
5.3
5.3
3.5
2.3
2.3
4.7
7.0
2.3
2.3
0
0
Musculoskeletal
Myalgia
2.6
0
Respiratory
Upper Respiratory Tract Infection
Sinusitis
Pharyngitis
5.3
3.5
2.6
7.0
0
0
Special Senses
Diplopia
Taste Perversion
Vision Abnormal
6.1
6.1
5.3
0
0
2.3
Children
Incidence in a Controlled Add-On Trial in Children with Lennox-Gastaut Syndrome:
Table 5 enumerates adverse events that occurred more than once among 31 pediatric patients who received felbamate up to 45 mg/kg/day or a maximum of 3600 mg/day. Reported adverse events were classified using standard WHO-based dictionary terminology.
Table 5. Children Treatment-Emergent Adverse Event Incidence in Controlled Add-On Lennox-Gastaut Trials Felbamate
(N = 31)
Placebo
(N = 27)
Body System/Event
%
%
Body as a Whole
Fever
Fatigue
Weight Decrease
Pain
22.6
9.7
6.5
6.5
11.1
3.7
0
0
Central Nervous System
Somnolence
Insomnia
Nervousness
Gait Abnormal
Headache
Thinking Abnormal
Ataxia
Urinary Incontinence
Emotional Lability
Miosis
48.4
16.1
16.1
9.7
6.5
6.5
6.5
6.5
6.5
6.5
11.1
14.8
18.5
0
18.5
3.7
3.7
7.4
0
0
Dermatological
Rash
9.7
7.4
Digestive
Anorexia
Vomiting
Constipation
Hiccup
Nausea
Dyspepsia
54.8
38.7
12.9
9.7
6.5
6.5
14.8
14.8
0
3.7
0
3.7
Hematologic
Purpura
Leukopenia
12.9
6.5
7.4
0
Respiratory
Upper Respiratory Tract Infection
Pharyngitis
Coughing
45.2
9.7
6.5
25.9
3.7
0
Special Senses
Otitis Media
9.7
0
Other Events Observed in Association with the Administration of Felbamate:
In the paragraphs that follow, the adverse clinical events, other than those in the preceding tables, that occurred in a total of 977 adults and 357 children exposed to felbamate and that are reasonably associated with its use are presented. They are listed in order of decreasing frequency. Because the reports cite events observed in open-label and uncontrolled studies, the role of felbamate in their causation cannot be reliably determined.
Events are classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; and rare events are those occurring in fewer than 1/1000 patients.
Event frequencies are calculated as the number of patients reporting an event divided by the total number of patients (N = 1,334) exposed to felbamate.
Body as a Whole: Frequent: Weight increase, asthenia, malaise, influenza-like symptoms; Rare: anaphylactoid reaction, chest pain substernal.
Cardiovascular: Frequent: Palpitation, tachycardia; Rare: supraventricular tachycardia.
Central Nervous System: Frequent: Agitation, psychological disturbance, aggressive reaction: Infrequent: hallucination, euphoria, suicide attempt, migraine.
Digestive: Frequent: SGOT increased; Infrequent: esophagitis, appetite increased; Rare: GGT elevated.
Hematologic: Infrequent: Lymphadenopathy, leukopenia, leukocytosis, thrombocytopenia, granulocytopenia; Rare: antinuclear factor test positive, qualitative platelet disorder, agranulocytosis.
Metabolic/Nutritional: Infrequent: Hypokalemia, hyponatremia, LDH increased, alkaline phosphatase increased, hypophosphatemia; Rare: creatinine phosphokinase increased.
Musculoskeletal: Infrequent: Dystonia.
Dermatological: Frequent: Pruritus; Infrequent: urticaria, bullous eruption; Rare: buccal mucous membrane swelling, Stevens-Johnson Syndrome.
Special Senses: Rare: Photosensitivity allergic reaction.
Postmarketing Adverse Event Reports:
Voluntary reports of adverse events in patients taking felbamate (usually in conjunction with other drugs) have been received since market introduction and may have no causal relationship with the drug(s). These include the following by body system:
Body as a Whole: neoplasm, sepsis, L.E. syndrome, SIDS, sudden death, edema, hypothermia, rigors, hyperpyrexia.
Cardiovascular: atrial fibrillation, atrial arrhythmia, cardiac arrest, torsade de pointes, cardiac failure, hypotension, hypertension, flushing, thrombophlebitis, ischemic necrosis, gangrene, peripheral ischemia, bradycardia, Henoch-Schönlein purpura (vasculitis).
Central & Peripheral Nervous System: delusion, paralysis, mononeuritis, cerebrovascular disorder, cerebral edema, coma, manic reaction, encephalopathy, paranoid reaction, nystagmus, choreoathetosis, extrapyramidal disorder, confusion, psychosis, status epilepticus, dyskinesia, dysarthria, respiratory depression, apathy, concentration impaired.
Dermatological: abnormal body odor, sweating, lichen planus, livedo reticularis, alopecia, toxic epidermal necrolysis.
Digestive: (Refer to WARNINGS) hepatitis, hepatic failure, G.I. hemorrhage, hyperammonemia, pancreatitis, hematemesis, gastritis, rectal hemorrhage, flatulence, gingival bleeding, acquired megacolon, ileus, intestinal obstruction, enteritis, ulcerative stomatitis, glossitis, dysphagia, jaundice, gastric ulcer, gastric dilatation, gastroesophageal reflux.
Fetal Disorders: fetal death, microcephaly, genital malformation, anencephaly, encephalocele.
Hematologic: (Refer to WARNINGS) increased and decreased prothrombin time, anemia, hypochromic anemia, aplastic anemia, pancytopenia, hemolytic uremic syndrome, increased mean corpuscular volume (mcv) with and without anemia, coagulation disorder, embolism-limb, disseminated intravascular coagulation, eosinophilia, hemolytic anemia, leukemia, including myelogenous leukemia, and lymphoma, including T-cell and B-cell lymphoproliferative disorders.
Metabolic/Nutritional: hypernatremia, hypoglycemia, SIADH, hypomagnesemia, dehydration, hyperglycemia, hypocalcemia.
Musculoskeletal: arthralgia, muscle weakness, involuntary muscle contraction, rhabdomyolysis.
Respiratory: dyspnea, pneumonia, pneumonitis, hypoxia, epistaxis, pleural effusion, respiratory insufficiency, pulmonary hemorrhage, asthma.
Special Senses: hemianopsia, decreased hearing, conjunctivitis.
Urogenital: menstrual disorder, acute renal failure, hepatorenal syndrome, hematuria, urinary retention, nephrosis, vaginal hemorrhage, abnormal renal function, dysuria, placental disorder.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Four subjects inadvertently received felbamate as adjunctive therapy in dosages ranging from 5400 mg/day to 7200 mg/day for durations between 6 and 51 days. One subject who received 5400 mg/day as monotherapy for one week reported no adverse experiences. Another subject attempted suicide by ingesting 12,000 mg of felbamate in a 12-hour period. The only adverse experiences reported were mild gastric distress and a resting heart rate of 100 bpm. No serious adverse reactions have been reported. General supportive measures should be employed if overdosage occurs. It is not known if felbamate is dialyzable.
-
DOSAGE AND ADMINISTRATION
Felbamate has been studied as monotherapy and adjunctive therapy in adults and as adjunctive therapy in children with seizures associated with Lennox-Gastaut syndrome. As felbamate is added to or substituted for existing AEDs, it is strongly recommended to reduce the dosage of those AEDs in the range of 20% to 33% to minimize side effects (see Drug Interactions subsection).
Dosage Adjustment in the Renally Impaired:
Felbamate should be used with caution in patients with renal dysfunction. In the renally impaired, starting and maintenance doses should be reduced by one-half (see CLINICAL PHARMACOLOGY/Pharmacokinetics and PRECAUTIONS). Adjunctive therapy with medications which affect felbamate plasma concentrations, especially AEDs, may warrant further reductions in felbamate daily doses in patients with renal dysfunction.
Adults (14 years of age and over)
The majority of patients received 3600 mg/day in clinical trials evaluating its use as both monotherapy and adjunctive therapy.
Monotherapy: (Initial therapy)
Felbamate has not been systematically evaluated as initial monotherapy. Initiate felbamate at 1200 mg/day in divided doses three or 4 times daily. The prescriber is advised to titrate previously untreated patients under close clinical supervision, increasing the dosage in 600 mg increments every 2 weeks to 2400 mg/day based on clinical response and thereafter to 3600 mg/day if clinically indicated.
Conversion to Monotherapy:
Initiate felbamate at 1200 mg/day in divided doses 3 or 4 times daily. Reduce the dosage of concomitant AEDs by one-third at initiation of felbamate therapy. At week 2, increase the felbamate dosage to 2400 mg/day while reducing the dosage of other AEDs up to an additional one-third of their original dosage. At week 3, increase the felbamate dosage up to 3600 mg/day and continue to reduce the dosage of other AEDs as clinically indicated.
Adjunctive Therapy:
Felbamate should be added at 1200 mg/day in divided doses 3 or 4 times daily while reducing present AEDs by 20% in order to control plasma concentrations of concurrent phenytoin, valproic acid, phenobarbital and carbamazepine and its metabolites. Further reductions of the concomitant AEDs dosage may be necessary to minimize side effects due to drug interactions. Increase the dosage of felbamate by 1200 mg/day increments at weekly intervals to 3600 mg/day. Most side effects seen during felbamate adjunctive therapy resolve as the dosage of concomitant AEDs is decreased.
Table 6. Dosage Table (adults) - * See Adjunctive and Conversion to Monotherapy sections.
Dosage reduction of concomitant AEDs
WEEK 1
REDUCE original dose by 20% to 33%*
WEEK 2
REDUCE original dose by up to an additional 1/3
WEEK 3
REDUCE as clinically indicated
Felbamate Dosage
1200 mg/day Initial dose
2400 mg/day
Therapeutic dosage range
3600 mg/day
Therapeutic dosage range
While the above felbamate conversion guidelines may result in a felbamate 3600 mg/day dose within 3 weeks, in some patients titration to a 3600 mg/day felbamate dose has been achieved in as little as 3 days with appropriate adjustment of other AEDs.
Children with Lennox-Gastaut Syndrome (Ages 2 to 14 years)
Adjunctive Therapy:
Felbamate should be added at 15 mg/kg/day in divided doses 3 or 4 times daily while reducing present AEDs by 20% in order to control plasma levels of concurrent phenytoin, valproic acid, phenobarbital, and carbamazepine and its metabolites. Further reductions of the concomitant AEDs dosage may be necessary to minimize side effects due to drug interactions. Increase the dosage of felbamate by 15 mg/kg/day increments at weekly intervals to 45 mg/kg/day. Most side effects seen during felbamate adjunctive therapy resolve as the dosage of concomitant AEDs is decreased.
-
HOW SUPPLIED
Felbamate Tablets, USP contain 400 mg or 600 mg of felbamate, USP.
The 400 mg tablet is white to off-white, modified capsule shaped, functionally scored, bisected tablet “FE 400” debossed on bisect side (upper) and “ALV550” on opposite side (bottom) of the tablet. They are available as follows:
NDC: 47781-627-01
bottles of 100 tabletsThe 600 mg tablet is white to off-white, modified capsule shaped, functionally scored, bisected tablet “FE 600” debossed on bisect side (upper) and “ALV551” on opposite side (bottom) of the tablet. They are available as follows:
NDC: 47781-630-01
bottles of 100 tabletsStore at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
PHARMACIST: Dispense a Medication Guide with each prescription.
To report SUSPECTED ADVERSE REACTIONS, contact Alvogen, Inc. at 1-866-770-3024 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PI267-00 Rev. 08/2018
-
PATIENT/PHYSICIAN ACKNOWLEDGMENT FORM
FELBAMATE TABLETS SHOULD NOT BE USED BY PATIENTS UNTIL THERE HAS BEEN A COMPLETE DISCUSSION OF THE RISKS.
All patients treated with felbamate tablets should acknowledge that they understand the risks and other information about felbamate tablets discussed below, and physicians should acknowledge this discussion.
IMPORTANT INFORMATION AND WARNING:
Felbamate tablets, taken by itself or with other prescription and/or non-prescription drugs, can result in a severe, potentially fatal blood abnormality ("aplastic anemia") and/or severe, potentially fatal liver damage.
PATIENT ACKNOWLEDGMENT:
Do not sign this form if there is anything you do not understand about the information you have received. Ask your doctor about anything you do not understand before you initial any of the items below or sign this form.
My [My son, daughter, ward ________________________________________'s] treatment with felbamate tablets has been personally explained to me by Dr._____________________________. The following points of information, among others, have been specifically discussed and made clear and I have had the opportunity to ask any questions concerning this information:
- 1. I, _______________________________________________________________ (Patient's Name), understand that felbamate tablets are used to treat certain types of seizures and my physician has told me that I have this type(s) of seizures;
- INITIALS: __________________________
- 2. I understand that felbamate tablets are being used because my seizures have not been satisfactorily treated with other antiepileptic drugs;
- INITIALS: __________________________
- 3. I understand that there is a serious risk that I could develop aplastic anemia and/or liver failure, both of which are potentially fatal, by using felbamate tablets;
- INITIALS: __________________________
- 4. I understand that there are no laboratory tests which will predict if I am at an increased risk for one of the potentially fatal conditions;
- INITIALS: __________________________
- 5. I understand that I should have the recommended blood work before my treatment with felbamate tablets has begun (baseline) and periodically thereafter as clinical judgement warrants. I understand that although this blood work may help detect if I develop one of these conditions, it may do so only after significant, irreversible and potentially fatal damage has already occurred;
- INITIALS: __________________________
- 6. If I am currently taking other antiepileptic drugs, I understand that the manufacturer of felbamate tablets recommends that the dosage of these other drugs be decreased by a certain amount when felbamate tablets are started; if my physician determines that this should not be done in my case, he/she has explained the reason(s) for this decision;
- INITIALS: __________________________
- 7. I understand that I must immediately report any unusual symptoms to Dr. _______________________ and be especially aware of any rashes, easy bruising, bleeding, sore throats, fever, and/or dark urine;
- INITIALS: __________________________
- 8. I understand that antiepileptic drugs such as felbamate tablets may increase the risk of suicidal thoughts and behavior. I understand that I must immediately report any unusual changes in mood or behavior, symptoms of depression or thoughts about self-harm to Dr. ___________.
- INITIALS: __________________________
Patient, Parent, or Guardian
Address
Telephone
PHYSICIAN STATEMENT:
I have fully explained to the patient, ___________________________________________, the nature and purpose of the treatment with felbamate tablets and the potential risks associated with that treatment. I have asked the patient if he/she has any questions regarding this treatment or the risks and have answered those questions to the best of my ability. I also acknowledge that I have read and understand the prescribing information.
_________________________________________________________________________
Physician DateAcknowledgment Form 627-00 Rev.08/20168
NOTE TO PHYSICIAN:
It is strongly recommended that you retain a signed copy of the Patient/Physician Acknowledgment Form with the patient's medical records.
SUPPLY OF PATIENT/PHYSICIAN ACKNOWLEDGMENT FORMS:
A supply of "Patient/Physician Acknowledgment" Forms as printed above is available by calling Alvogen, Inc. at 1-866-770-3024. Permission to use the above Patient/Physician Acknowledgment Form by photocopy reproduction is also hereby granted by Alvogen, Inc.
-
Medication Guide
Medication Guide
FELBAMATE TABLETS, USP
(fel bam' ate)
400 mg and 600 mgRead this Medication Guide before you start taking felbamate tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about felbamate tablets?
Do not stop taking felbamate tablets without first talking to your healthcare provider. Stopping felbamate tablets suddenly can cause serious problems.
Felbamate tablets can cause serious side effects, including:
- 1. Felbamate tablets may cause serious blood problems that may be life-threatening.
-
Call your healthcare provider right away if you have any of the following symptoms:
- Fever, sore throat or other infections that come and go or do not go away
- Frequent infections or an infection that does not go away
- Easy bruising
- Red or purple spots on your body
- Bleeding gums or nose bleeds
- Severe fatigue or weakness
- 2.
Liver problems that may be life-threatening. Call your healthcare provider right away if you have any of these symptoms:
- yellowing of your skin or the whites of your eyes (jaundice)
- dark urine
- nausea or vomiting
- loss of appetite
- pain on the right side of your stomach (abdomen)
- 3. Like other antiepileptic drugs, felbamate tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
- 4.
Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop felbamate tablets without first talking to a healthcare provider.
Stopping felbamate tablets suddenly can cause serious problems. You should talk to your healthcare provider before stopping. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
What are felbamate tablets?
Felbamate tablets are a prescription medicine used when other treatments have failed in:
-
adults alone or with other medicines to treat:
- o partial seizures with and without generalization
-
children with other medicines to treat:
- o seizures associated with Lennox-Gastaut syndrome
Who should not take felbamate tablets?
Do not take felbamate tablets if you:
- are allergic to felbamate, carbamates or any of the ingredients in felbamate tablets. See the end of this Medication Guide for a complete list of ingredients in felbamate tablets.
- have or have had blood problems
- have or have had liver problems
What should I tell my healthcare provider before taking felbamate tablets?
Before you take felbamate tablets, tell your healthcare provider if you:
- have kidney problems
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have any other medical conditions
-
are pregnant or plan to become pregnant. It is not known if felbamate tablets can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking felbamate tablets. You and your healthcare provider will decide if you should take felbamate tablets while you are pregnant.
- o If you become pregnant while taking felbamate tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic medicine during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. Felbamate may pass into your breast milk. You and your healthcare provider should decide if you should take felbamate tablets while you breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking felbamate tablets with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take felbamate tablets?
- Take felbamate tablets exactly as your healthcare provider tells you. Your healthcare provider will tell you how much felbamate to take and when to take it.
- Your healthcare provider may change your dose of felbamate. Do not change your dose of felbamate without talking to your healthcare provider.
- Because of the risk of serious blood and liver problems, your healthcare provider may do blood tests before you start and while you take felbamate tablets.
- If you take too much felbamate, call your healthcare provider or local Poison Control Center right away.
- Do not stop felbamate tablets without first talking to your healthcare provider.
What should I avoid while taking felbamate tablets?
- Felbamate tablets can cause drowsiness and dizziness. Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking felbamate tablets, until you talk with your doctor. Taking felbamate tablets with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
What are the possible side effects of felbamate tablets?
See “What is the most important information I should know about felbamate tablets?”
Felbamate tablets may cause serious side effects including:
The most common side effects of felbamate tablets include:
- weight loss
- vomiting
- trouble sleeping
- nausea
- dizziness
- sleepiness
- headache
- double-vision
- changes in the way that food tastes
These are not all the possible side effects of felbamate tablets. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store felbamate tablets?
- Store felbamate tablets at 20° to 25°C (68° to 77°F).
Keep felbamate tablets and all medicines out of the reach of children.
General information about felbamate tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use felbamate tablets for a condition for which it was not prescribed. Do not give felbamate tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about felbamate tablets. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about felbamate tablets that is written for health professionals.
What are the ingredients in felbamate tablets?
Active Ingredient: felbamate, USP
Inactive Ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose.
For more information, call Alvogen, Inc. at 1-866-770-3024.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Product of India
Distributed by:
Alvogen, Inc.
Pine Brook, NJ 07058 USAPL627-00Rev. 08/2018
- PRINCIPAL DISPLAY PANEL - 400 mg
- PRINCIPAL DISPLAY PANEL - 600 mg
-
INGREDIENTS AND APPEARANCE
FELBAMATE
felbamate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 47781-627 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FELBAMATE (UNII: X72RBB02N8) (FELBAMATE - UNII:X72RBB02N8) FELBAMATE 400 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape CAPSULE (modified capsule) Size 16mm Flavor Imprint Code FE;400;ALV550 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47781-627-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/24/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204595 12/01/2018 FELBAMATE
felbamate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 47781-630 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FELBAMATE (UNII: X72RBB02N8) (FELBAMATE - UNII:X72RBB02N8) FELBAMATE 600 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape CAPSULE (modified capsule) Size 19mm Flavor Imprint Code FE;600;ALV551 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47781-630-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/23/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204595 12/01/2018 Labeler - Alvogen Inc. (008057330) Establishment Name Address ID/FEI Business Operations Norwich Pharmaceuticals, Inc. 132218731 analysis(47781-627, 47781-630) , manufacture(47781-627, 47781-630) , pack(47781-627, 47781-630)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.