EXTRALASTING LIQUID FOUNDATION- titanium dioxide lotion
Extralasting by
Drug Labeling and Warnings
Extralasting by is a Otc medication manufactured, distributed, or labeled by New Avon LLC, Avon Products, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
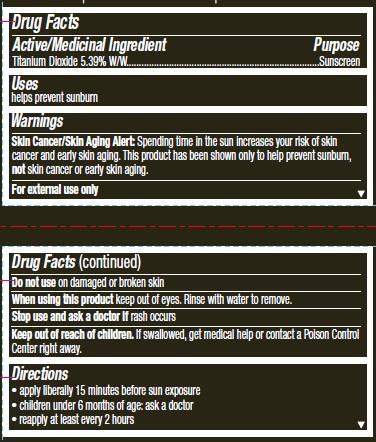
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive/non-medicinal ingredients: Water/Eau, Cyclopentasiloxane, Dimethicone, Dipropylene Glycol, Glycerin, PEG-10 Dimethicone, Nylon-12, Polymethyl Methacrylate, Trisiloxane, Methyl Trimethicone, Acrylates/Dimethicone Copolymer, Sodium Chloride, Quaternium-90 Bentonite, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Aluminum Hydroxide, Caprylyl Glycol, Stearic Acid, Phenoxyethanol, Tetrasodium EDTA, Propylene Carbonate, Hexylene Glycol, Cellulose, Trifluoromethyl C1-4 Alkyl Dimethicone, Parfum/Fragrance, Triethoxycaprylylsilane, Glyceryl Rosinate, Octyldodecyl Myristate. May Contain: Titanium Dioxide/CI 77891, Iron Oxides.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXTRALASTING LIQUID FOUNDATION
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10096-0232 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 53.9 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10096-0232-2 1 in 1 CARTON 1 NDC: 10096-0232-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/15/2010 Labeler - New Avon LLC (080143520) Establishment Name Address ID/FEI Business Operations Avon Products, Inc. 005149471 manufacture(10096-0232)
Trademark Results [Extralasting]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXTRALASTING 85054709 3907636 Live/Registered |
AVON NA IP LLC 2010-06-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.