4167 FIRST AID KIT- 4167 first aid kit
4167 First Aid Kit by
Drug Labeling and Warnings
4167 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
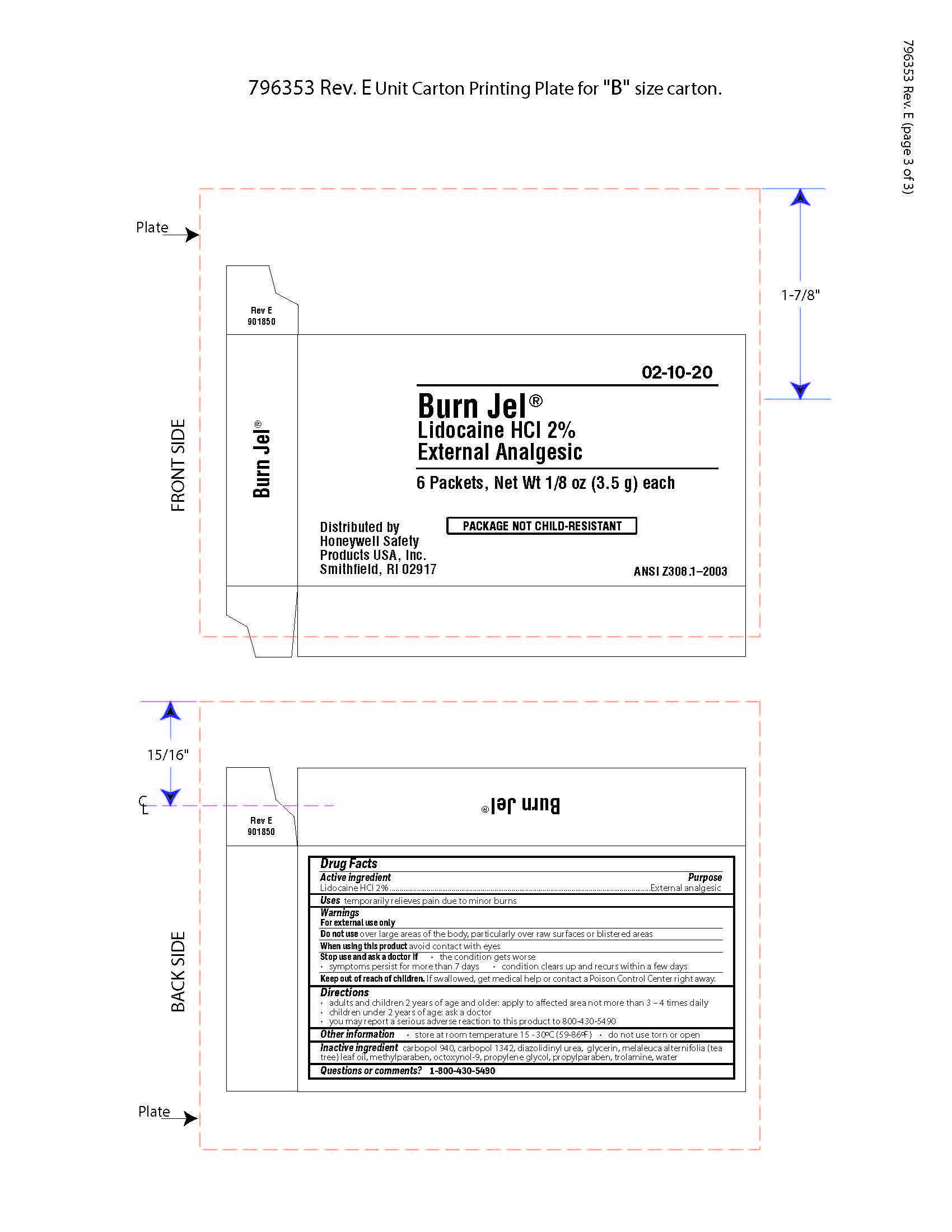
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn JEl Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
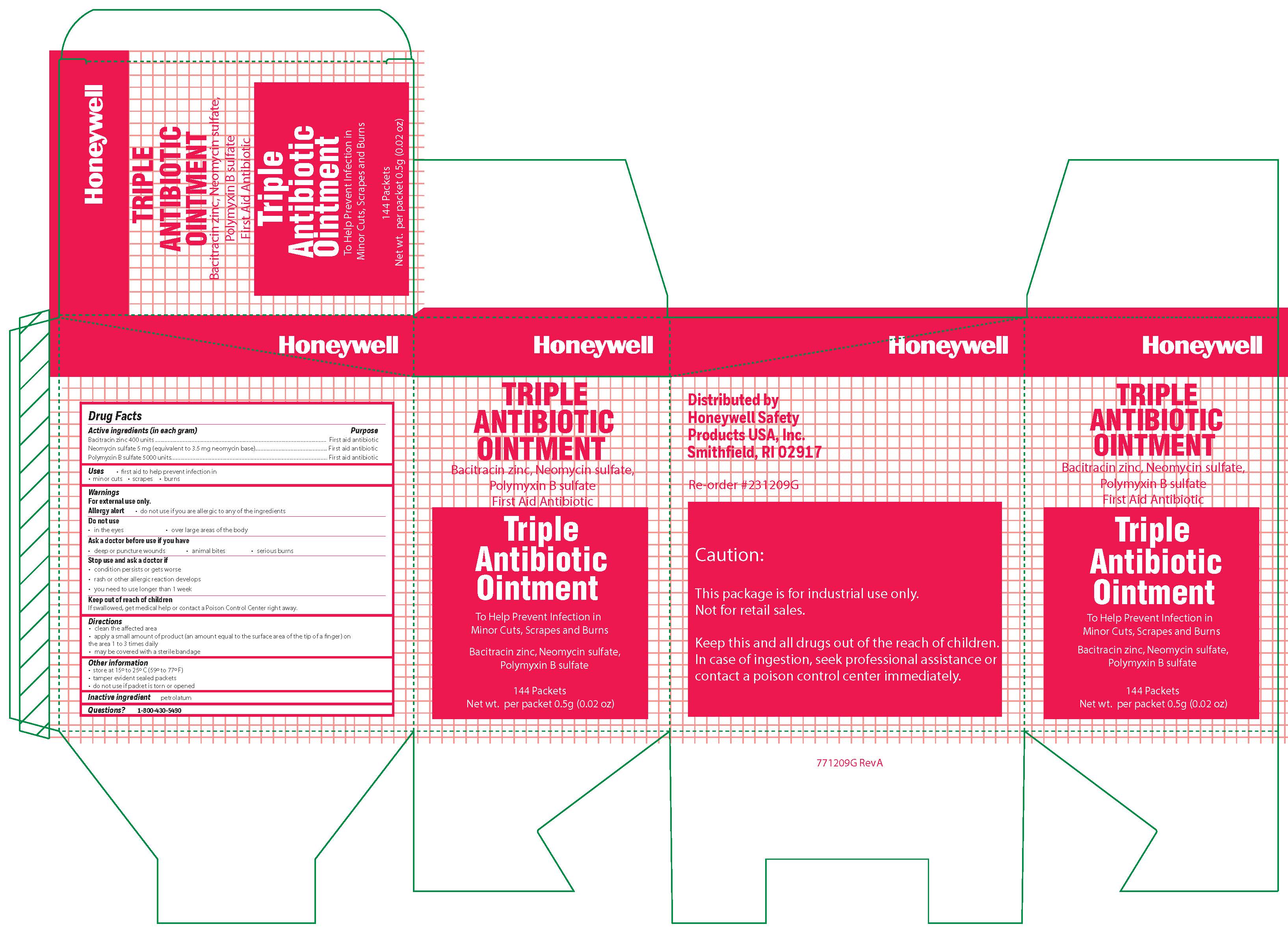
- Triple Active ingredient
- Triple Purpose
- Triple Uses
-
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
- Triple Questions?
- BZK Wipe Active ingredient
- BZK Wipe Purpose
- BzK Wipe Uses
-
BZK Wipe
Warnings
For external use onlyDo not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Wipe Directions
- BZK Wipe Other information
- BZK Wipe Inactive ingredient
- BZK Wipe Questions
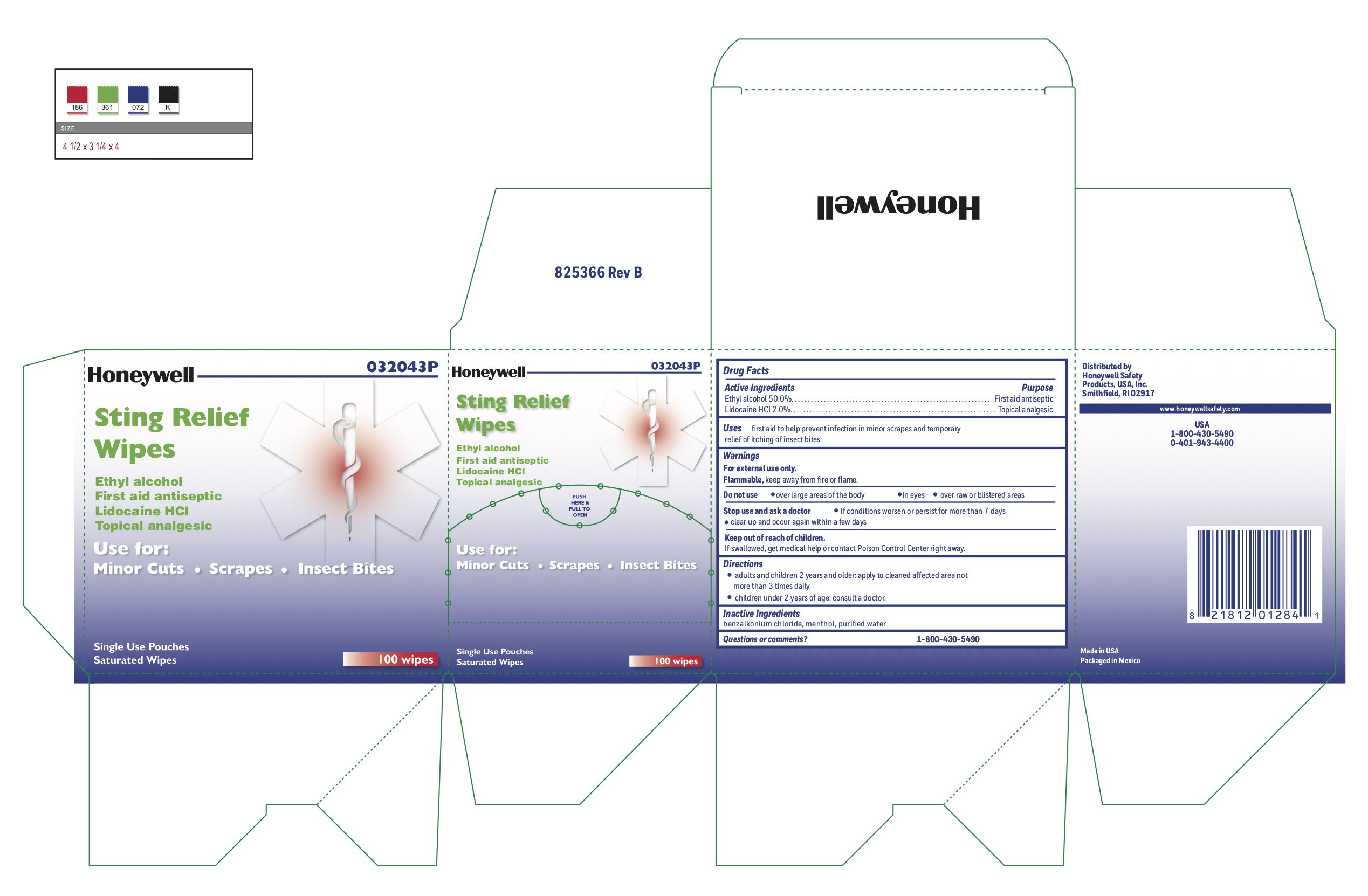
- Sting Relief Active ingredient (in each wipe)
- Sting Relief Purposse
- Sting Relief Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting Relief Questions or Comments?
- PVP Ampule Inactive ingredients
- PVP Ampule Questions
- Eyewash Active ingredient
- Eyewassh Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyeash Directions
- Eyewash Inactive ingredients
- Eyeash Questions
-
4167
6824PE Kit Contents
2 TRIPLE ANTIBIOTIC 10 PER
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE COMPRESS, 1728 SQ IN 1
2 INSTANT COLD PACK 4" X 6"
2 ADHESIVE BDG,PLSTIC,1"X3"16PER
2 BURN JEL 1/8 OZ, 6 PER
2 NITRILE GLOVES 2PR BBP
4 ANTIMCRBL ANTSPTC TWLETTS
1 CPR MICROSHIELD DOUBLE UNIT
1 1 OZ, BUFF EYEWASH
1 F. A. INST CHART SM (INDIVIDUAL LBL)
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
1 LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 24 UN WHITE 01
1 2 1/4 X 3 1/2 BANDS 6
1 STING Relief SWAB 10
1 GAUZE PADS 3"X3" 4/BX
1 SCISSOR & FORCEP 1 EA
- Burn Jel Principal Display Panel
- Triple Principal Display Panel
- BZK Wipe Principal Display Panel
- Sting Relief Principal Display Panel
- Eyewash Principal Display Panel
- 4167 Kit Label 6824PE
-
INGREDIENTS AND APPEARANCE
4167 FIRST AID KIT
4167 first aid kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4167 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4167-01 1 in 1 KIT 09/13/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKET 21 g Part 2 1 BOTTLE 30 mL Part 3 20 PACKET 18 g Part 4 4 PACKET 5.6 mL Part 5 10 POUCH 4 mL Part 1 of 5 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) DIPROPYLENE GLYCOL (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/19/2018 Part 2 of 5 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 3 of 5 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC: 0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 4 of 5 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/22/2017 Part 5 of 5 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0733-00 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/13/2018 Labeler - Honeywell Safety Products USA, Inc. (079287321) Registrant - Honeywell Safety Products USA, Inc. (079287321) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc 079287321 pack(0498-4167) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0203, 0498-0750) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0501) Establishment Name Address ID/FEI Business Operations Safetec of America Inc 874965262 manufacture(0498-0733)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.