SAINT External Pain Relieving Bandage
SAINT External Pain Relieving Bandage by
Drug Labeling and Warnings
SAINT External Pain Relieving Bandage by is a Otc medication manufactured, distributed, or labeled by Saint Medicine, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
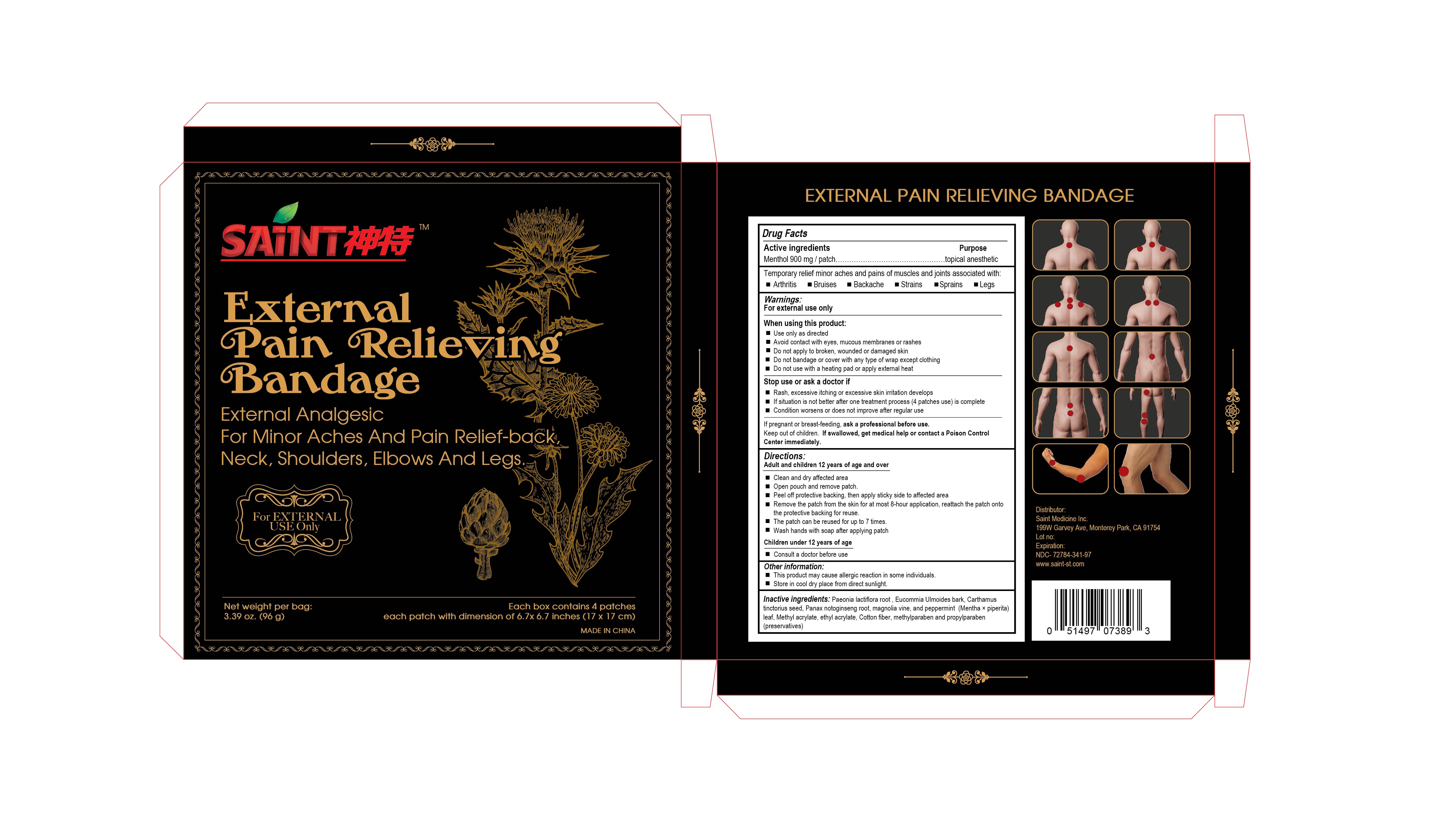
SAINT EXTERNAL PAIN RELIEVING BANDAGE- menthol patch
Saint Medicine, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SAINT External Pain Relieving Bandage
Uses
Temporary relief minor aches and pains of muscles and joints associated with:
Arthritis Bruises Backache Strains Sprains Legs
Warnings:
For external use only
When using this product:
Use only as directed
Avoid contact with eyes, mucous membranes or rashes
Do not apply to broken, wounded or damaged skin
Do not bandage or cover with any type of wrap except clothing
Do not use with a heating pad or apply external heat
Stop use or ask a doctor if
Rash, excessive itching or excessive skin irritation develops
If situation is not better after one treatment process (4 patches use) is complete
Condition worsens, or does not improve after regular use
If pregnant or breast-feeding, ask a professional before use.
Directions:
Adult and children 12 years of age and over
Clean and dry affected area
Open pouch and remove patch.
Peel off protective backing, then apply sticky side to affected area.
Remove the patch from the skin for at most 8-hour application, reattach the patch onto the protective backing for reuse.
The patch can be reused for up to 7 times
Wash hands with soap after applying patch
Children under 12 years of age
Consult a doctor before use
Other information:
This product may cause allergic reaction in some individuals.
Store in cool dry place from direct sunlight.
Inactive ingredients:
Paeonia lactiflora root, Eucommia Ulmoides bark, Carthamus tinctorius seed, Panax notoginseng root, Magnolia vine, Peppermint (Mentha × piperita) leaf, Methyl acrylate, Ethyl acrylate, Cotton fiber, Methylparaben, Propylparaben (preservatives)
| SAINT EXTERNAL PAIN RELIEVING BANDAGE
menthol patch |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Saint Medicine, Inc (081508032) |