MESALAMINE RECTAL- mesalamine suppository
Mesalamine Rectal by
Drug Labeling and Warnings
Mesalamine Rectal by is a Prescription medication manufactured, distributed, or labeled by Amring Pharmaceuticals Inc., Confab Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MESALAMINE RECTAL SUPPOSITORIES safely and effectively. See full prescribing information for MESALAMINE RECTAL SUPPOSITORIES.
MESALAMINE suppositories, for rectal use
Initial U.S. Approval: 1987
INDICATIONS AND USAGE
Mesalamine Rectal Suppository is an aminosalicylate indicated in adults for the treatment of mildly to moderately active ulcerative proctitis. (1)
DOSAGE AND ADMINISTRATION
Dosage
The recommended adult dosage is 1000 mg administered rectally once daily at bedtime for 3 to 6 weeks. Safety and effectiveness beyond 6 weeks have not been established. (2)Administration Instructions (2):
- Evaluate renal function prior to initiation of Mesalamine Rectal Suppository and periodically while on therapy (2.1, 5.1)
- Do not cut or break the suppository.
- Retain the suppository for one to three hours or longer, if possible.
- Mesalamine Rectal Suppositories will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Keep Mesalamine Rectal Suppository away from these surfaces to prevent staining.
DOSAGE FORMS AND STRENGTHS
Suppository: 1000 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Renal Impairment: Evaluate the risks and benefits in patients with known renal impairment or taking nephrotoxic drugs; monitor renal function (5.1, 7.1, 8.6)
- Mesalamine-Induced Acute Intolerance Syndrome: Symptoms may be difficult to distinguish from an exacerbation of ulcerative colitis; monitor for worsening symptoms; discontinue treatment if acute intolerance syndrome is suspected. (5.2)
- Hypersensitivity Reactions, including Myocarditis and Pericarditis: Evaluate patients immediately and discontinue if a hypersensitivity reaction is suspected. (5.3)
- Hepatic Failure: Evaluate the risks and benefits in patients with known liver impairment. (5.4)
- Interaction with Laboratory Test for Urinary Normetanephrine: Spuriously elevated test results may occur with liquid chromatography with electrochemical detection in patients receiving Mesalamine Rectal Suppository; use alternative, selective assay for normetanephrine. (5.5, 7.3)
ADVERSE REACTIONS
The most common adverse reactions (≥ 1%) are: dizziness, rectal pain, fever, rash, acne and colitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amring Pharmaceuticals Inc. at 1-844-Amring1 (1-844-267-4641) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Geriatric Patients: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
5.2 Mesalamine-Induced Acute Intolerance Syndrome
5.3 Hypersensitivity Reactions
5.4 Hepatic Failure
5.5 Interaction with Laboratory Test for Urinary Normetanephrine
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7. DRUG INTERACTIONS
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
7.2 Azathioprine or 6-Mercaptopurine
7.3 Urinary Normetanephrine Measurements
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
Dosage
The recommended dosage of Mesalamine Rectal Suppository in adults is 1000 mg administered rectally once daily at bedtime for 3 to 6 weeks depending on symptoms and sigmoidoscopic findings. Safety and effectiveness of Mesalamine Rectal Suppository beyond 6 weeks have not been established.Administration Instructions:
- Evaluate renal function prior to initiation of Mesalamine Rectal Suppository therapy and periodically while on therapy.
- Do not cut or break the suppository.
- Retain the suppository for one to three hours or longer, if possible.
- If a dose of Mesalamine Rectal Suppository is missed, administer as soon as possible, unless it is almost time for next dose. Do not use two Mesalamine Rectal Suppositories at the same time to make up for a missed dose.
- Mesalamine Rectal Suppositories will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Keep Mesalamine Rectal Suppository away from these surfaces to prevent staining.
- 3. DOSAGE FORMS AND STRENGTHS
-
4. CONTRAINDICATIONS
Mesalamine Rectal Suppository is contraindicated in patients with known or suspected hypersensitivity to salicylates or aminosalicylates or to any ingredients in the suppository vehicle [see Warnings and Precautions (5.3), Adverse Reactions (6.2), and Description (11)].
-
5. WARNINGS AND PRECAUTIONS
5.1 Renal Impairment
Renal impairment, including minimal change nephropathy, acute and chronic interstitial nephritis, and renal failure, has been reported in patients given products such as Mesalamine Rectal Suppository that contain mesalamine or are converted to mesalamine [see Adverse Reactions (6.2)].
Evaluate renal function prior to initiation of Mesalamine Rectal Suppository therapy and periodically while on therapy.
Evaluate the risks and benefits of using Mesalamine Rectal Suppository in patients with known renal impairment or a history of renal disease or taking concomitant nephrotoxic drugs. In animal studies, the kidney was the principal organ for toxicity [see Drug Interactions (7.1), Use in Specific Populations (8.6) and Nonclinical Toxicology (13.2)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, and sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with Mesalamine Rectal Suppository.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some patients may have a similar reaction to Mesalamine Rectal Suppository or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue Mesalamine Rectal Suppository if an alternative etiology for the signs and symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered other products containing mesalamine. Evaluate the risks and benefits of using Mesalamine Rectal Suppository in patients with known liver impairment.
5.5 Interaction with Laboratory Test for Urinary Normetanephrine
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection, because of the similarity in the chromatograms of normetanephrine and mesalamine's main metabolite, N-acetylaminosalicylic acid. Consider an alternative, selective assay for normetanephrine.
-
6. ADVERSE REACTIONS
The most serious adverse reactions seen in Mesalamine Rectal Suppository clinical trials or with other products that contain or are metabolized to mesalamine are:
- Renal Impairment [see Warnings and Precautions (5.1)]
- Mesalamine-Induced Acute Intolerance Syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Failure [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions in adult patients with mildly to moderately active ulcerative proctitis in double-blind, placebo-controlled trials are summarized in the Table 1 below.
Table 1: Adverse Reactions Occurring In More Than 1% of Mesalamine Suppository Treated Patients (Comparison to Placebo) Symptom
Mesalamine
(n = 177)
Placebo
(n = 84)
N
%
N
%
Dizziness
5
3
2
2.4
Rectal Pain
3
1.8
0
0
Fever
2
1.2
0
0
Rash
2
1.2
0
0
Acne
2
1.2
0
0
Colitis
2
1.2
0
0
In a multicenter, open-label, randomized, parallel group study in 99 patients comparing the Mesalamine Rectal Suppository 1000 mg suppository administered nightly to that of the mesalamine 500 mg suppository twice daily. The most common adverse reactions in both groups were headache (14%), flatulence (5%), abdominal pain (5%), diarrhea (3%), and nausea (3%). Three (3) patients discontinued medication because of an adverse reaction; one of these adverse reactions (headache) was deemed possibly related to study medication. The recommended dosage of Mesalamine Rectal Suppository is 1000 mg administered rectally once daily at bedtime [see Dosage and Administration (2)].
6.2 Postmarketing Experience
In addition to the adverse reactions reported above in clinical trials involving Mesalamine Rectal Suppository, the adverse reactions listed below have been identified during post-approval use of Mesalamine Rectal Suppository and other mesalamine-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Body as a Whole: drug fever, fatigue, lupus-like syndrome, medication residue
- Cardiac Disorders: myocarditis, pericarditis, pericardial effusion [see Warnings and Precautions (5.3)]
- Endocrine: Nephrogenic diabetes insipidus
- Eye disorders: eye swelling
- Gastrointestinal Disorders: abdominal cramps, abdominal distension, anal pruritus, anorectal discomfort, constipation, feces discolored, flatulence, frequent bowel movements, gastrointestinal bleeding, mucus stools, nausea, painful defecation, pancreatitis, proctalgia, rectal discharge, rectal tenesmus, stomach discomfort, vomiting
- Hepatic Disorders: cholestatic jaundice, hepatitis, jaundice, Kawasaki-like syndrome including changes in liver enzymes, liver necrosis, liver failure
- Hematologic Disorders: agranulocytosis, aplastic anemia, thrombocytopenia
- Neurological/Psychiatric Disorders: Guillain-Barre syndrome, peripheral neuropathy, transverse myelitis, intracranial hypertension
- Renal Disorders: interstitial nephritis, renal failure, minimal change nephropathy [see Warnings and Precautions (5.1)]
- Respiratory, Thoracic and Mediastinal Disorders: hypersensitivity pneumonitis (including allergic alveolitis, eosinophilic pneumonitis, interstitial pneumonitis)
- Skin and Subcutaneous Tissue Disorder: alopecia, erythema, erythema nodosum, pruritus, psoriasis, pyoderma gangrenosum, urticaria
- Urogenital: reversible oligospermia
-
7. DRUG INTERACTIONS
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine- related adverse reactions [see Warnings and Precautions (5.1)].
7.2 Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine may increase the risk for blood disorders. If concomitant use of Mesalamine Rectal Suppository and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
7.3 Urinary Normetanephrine Measurements
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection, because of the similarity in the chromatograms of normetanephrine and mesalamine's main metabolite, N-acetylaminosalicylic acid. Consider an alternative, selective assay for normetanephrine [see Warnings and Precautions (5.5)].
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data on mesalamine use in pregnant women are insufficient to inform a drug-associated risk. No evidence of teratogenicity was observed in rats or rabbits when treated during gestation with orally administered mesalamine at doses greater than the recommended human intra-rectal dose [see Data].The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Reproduction studies have been performed in rats at oral doses up to 320 mg/kg/day (about 1.7 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) and in rabbits at oral doses up to 495 mg/kg/day (about 5.4 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) following administration during the period of organogenesis, and have revealed no evidence of impaired fertility or harm to the fetus due to mesalamine.8.2 Lactation
Risk Summary
Mesalamine and its N-acetyl metabolite are present in human milk in undetectable to small amounts [see Data]. There are limited reports of diarrhea in breastfed infants. There is no information on the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of Mesalamine Rectal Suppository to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Mesalamine Rectal Suppository and any potential adverse effects on the breastfed child from Mesalamine Rectal Suppository or from the underlying maternal conditions.Clinical Considerations
Monitor breastfed infants for diarrhea.Data
In published lactation studies, maternal mesalamine doses from various oral and rectal formulations and products ranged from 500 mg to 3 g daily. The concentration of mesalamine in milk ranged from non-detectable to 0.11 mg/L. The concentration of the N-acetyl-5- aminosalicylic acid metabolite ranged from 5 to 18.1 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.017 mg/kg/day of mesalamine and 0.75 to 2.72 mg/kg/day of N-acetyl-5-aminosalicylic acid.8.4 Pediatric Use
The safety and effectiveness of Mesalamine Rectal Suppository in pediatric patients for the treatment of mildly to moderately active ulcerative proctitis have not been established.
Information describing a clinical study in which efficacy was not demonstrated in pediatric patients ages 5 to 17 years is approved for Forest Laboratories, LLC’s CANASA (mesalamine) rectal suppositories. However, due to Forest Laboratories, LLC’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
Clinical trials of Mesalamine Rectal Suppository did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Systemic exposures are increased in elderly subjects [See Clinical Pharmacology (12.3)]. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia and pancytopenia) in patients receiving mesalamine-containing products such as Mesalamine Rectal Suppository who were 65 years or older compared to younger patients. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with Mesalamine Rectal Suppository. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concurrent disease or other drug therapy in elderly patients should be considered when prescribing Mesalamine Rectal Suppository [see Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on Mesalamine Rectal Suppository therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1), Drug Interactions (7.1) and Adverse Reactions (6.2)].
- 10. OVERDOSAGE
-
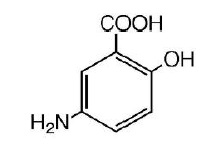
11. DESCRIPTION
The active ingredient in Mesalamine Rectal Suppository 1000 mg suppositories for rectal use is mesalamine, also known as mesalazine or 5- aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid, and is classified as an anti-inflammatory drug. Each Mesalamine Rectal Suppository contains 1000 mg of mesalamine (USP) in a base of Hard Fat, NF.
The empirical formula is C7H7NO3, representing a molecular weight of 153.14. The structural formula is:
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of mesalamine is not fully understood, but appears to be topical rather than systemic. Although the pathology of inflammatory bowel disease is uncertain, both prostaglandins and leukotrienes have been implicated as mediators of mucosal injury and inflammation.
12.3 Pharmacokinetics
Absorption
Mesalamine (5-ASA) administered as a rectal suppository is variably absorbed. In patients with ulcerative colitis treated with mesalamine 500 mg rectal suppositories, administered once every eight hours for six days, the mean mesalamine peak plasma concentration (Cmax) was 353 ng/mL (CV=55%) following the initial dose and 361 ng/mL (CV=67%) at steady state. The mean minimum steady state plasma concentration (Cmin) was 89 ng/mL (CV=89%). Absorbed mesalamine does not accumulate in the plasma.Distribution
Mesalamine administered as a rectal suppository distributes in rectal tissue to some extent.Elimination
In patients with ulcerative proctitis treated with mesalamine 500 mg as a rectal suppository every 8 hours for 6 days, the mean elimination half-life was 5 hours (CV=73%) for 5-ASA and 5 hours (CV=63%) for N-acetyl-5-ASA, the active metabolite, following the initial dose. At steady state, the mean elimination half-life was 7 hours for both 5-ASA and N-acetyl-5-ASA (CV=102% for 5- ASA and 82% for N-acetyl-5-ASA).Metabolism
The absorbed mesalamine is extensively metabolized, mainly to N-acetyl-5-ASA in the liver and in the gut mucosal wall. In patients with ulcerative colitis treated with one mesalamine 500 mg rectal suppository every eight hours for six days, the peak concentration (Cmax) of N-acetyl-5-ASA ranged from 467 ng/mL to 1399 ng/mL following the initial dose and from 193 ng/mL to 1304 ng/mL at steady state.Excretion
Mesalamine is eliminated from plasma mainly by urinary excretion, predominantly as N-acetyl-5-ASA. In patients with ulcerative proctitis treated with mesalamine 500 mg as a rectal suppository every 8 hours for 6 days, 12% or less of the dose was eliminated in urine as unchanged 5-ASA and 8% to 77% was eliminated as N-acetyl-5-ASA following the initial dose. At steady state, 11% or less of the dose was eliminated in the urine as unchanged 5-ASA and 3% to 35% was eliminated as N-acetyl-5-ASA. -
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mesalamine caused no increase in the incidence of neoplastic lesions over controls in a two-year study of Wistar rats fed up to 320 mg/kg/day of mesalamine admixed with diet (about 1.7 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area).
Mesalamine was not mutagenic in the Ames test, the mouse lymphoma cell (TK+/-) forward mutation test, or the mouse micronucleus test.
No effects on fertility or reproductive performance of the male and female rats were observed at oral mesalamine doses up to 320 mg/kg/day (about 1.7 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area).
13.2 Animal Toxicology and/or Pharmacology
Toxicology studies of mesalamine were conducted in rats, mice, rabbits and dogs, and the kidney was the main target organ of toxicity. In rats, adverse renal effects were observed at a single oral dose of 600 mg/kg (about 3.2 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) and at intravenous doses of >214 mg/kg (about 1.2 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area). In a 13-week oral gavage toxicity study in rats, papillary necrosis and/or multifocal tubular injury were observed in males receiving 160 mg/kg (about 0.86 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) and in both males and females at 640 mg/kg (about 3.5 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area). In a combined 52-week toxicity and 127-week carcinogenicity study in rats, degeneration of the kidneys and hyalinization of basement membranes and Bowman’s capsule were observed at oral doses of 100 mg/kg/day (about 0.54 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) and above. In a 14- day rectal toxicity study of mesalamine suppositories in rabbits, intra-rectal doses up to 800 mg/kg (about 8.6 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) was not associated with any adverse effects. In a six-month oral toxicity study in dogs, doses of 80 mg/kg (about 1.4 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) and higher caused renal pathology similar to that described for the rat. In a rectal toxicity study of mesalamine suppositories in dogs, a dose of 166.6 mg/kg (about 3 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) produced chronic nephritis and pyelitis. In the 12-month eye toxicity study in dogs, keratoconjunctivitis sicca (KCS) occurred at oral doses of 40 mg/kg (about 0.72 times the recommended human intra-rectal dose of Mesalamine Rectal Suppository, based on body surface area) and above.
-
14. CLINICAL STUDIES
Two double-blind, placebo-controlled, multicenter trials of mesalamine suppositories were conducted in North America in adult patients with mildly to moderately active ulcerative proctitis. The regimen in Study 1 was a 500 mg mesalamine suppository administered rectally three times daily and in Study 2 was a 500 mg mesalamine suppository administered rectally twice daily. In both trials, patients had an average extent of proctitis (upper disease boundary) of approximately 10 cm and approximately 80% of patients had multiple prior episodes of proctitis. A total of 173 patients were evaluated (Study 1, N=79; Study 2, N=94), of which 89 patients received mesalamine, and 84 patients received placebo. The mean age of patients was 39 years (range 17 to 73 years), 60% were female, and 97% were white.
The primary measures of efficacy were clinical disease activity index (DAI) and histologic evaluations in both trials. The DAI is a composite index reflecting rectal bleeding, stool frequency, mucosal appearance at endoscopy, and a physician’s global assessment of disease. Patients were evaluated clinically and sigmoidoscopically after 3 and 6 weeks of treatment.
Compared to placebo, mesalamine suppositories were statistically (p<0.01) superior to placebo in both trials with respect to improvement in stool frequency, rectal bleeding, mucosal appearance, disease severity, and overall disease activity after 3 and 6 weeks of treatment. The effectiveness of mesalamine suppositories was statistically significant irrespective of sex, extent of proctitis, duration of current episode, or duration of disease.
An additional multicenter, open-label, randomized, parallel group study in 99 patients diagnosed with mildly to moderately ulcerative proctitis compared 1000 mg Mesalamine Rectal Suppository administered rectally once daily at bedtime (N=35) to 500 mg mesalamine suppository administered rectally twice daily, in the morning and at bedtime (N=46), for 6 weeks.
The primary measures of efficacy included the clinical disease activity index (DAI) and histologic evaluations. Patients were evaluated clinically and sigmoidoscopically at 3 and 6 weeks of treatment.
The efficacy at 6 weeks was not different between the treatment groups. Both were effective in the treatment of ulcerative proctitis and resulted in a significant decrease at 6 weeks in DAI: in the mesalamine 500 mg twice daily group, the mean DAI value decreased from 6.6 to 1.6, and in the 1000 mg at bedtime group, the mean DAI value decreased from 6.2 to 1.3, which represents a decrease of greater than 75% in both groups. After 6 weeks of treatment, a DAI score of less than 3 was achieved in 78% of patients in the mesalamine 500 mg twice daily group and 86% of patients in the Mesalamine Rectal Suppository 1000 mg once daily group. The recommended dosage of Mesalamine Rectal Suppository is 1000 mg administered rectally once daily at bedtime [see Dosage and Administration (2)].
-
16. HOW SUPPLIED/STORAGE AND HANDLING
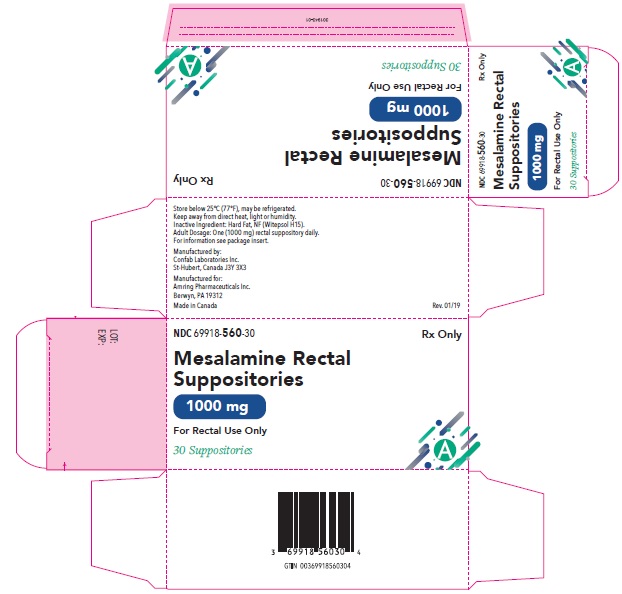
Mesalamine Rectal Suppositories 1000 mg for rectal administration are available as torpedo shaped, white to beige suppositories containing 1000 mg mesalamine supplied in boxes of 30 (NDC: 69918-560-30).
Store below 25ºC (77ºF), may be refrigerated. Keep away from direct heat, light or humidity.
-
17. PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information)
Administration Advise patients:
- Do not cut or break the suppository.
- Retain the suppository for one to three hours or longer, if possible.
- If a dose of Mesalamine Rectal Suppository is missed, administer as soon as possible, unless it is almost time for next dose. Do not use two Mesalamine Rectal Suppositories at the same time to make up for a missed dose.
- Mesalamine Rectal Suppositories will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Keep Mesalamine Rectal Suppository away from these surfaces to prevent staining.
Renal Impairment
Inform patients that Mesalamine Rectal Suppository may decrease their renal function, especially if they have known renal impairment or are taking nephrotoxic drugs, including NSAIDs, and periodic monitoring of renal function will be performed while they are on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)].Mesalamine-Induced Acute Intolerance Syndrome and Other Hypersensitivity Reactions
Inform patients of the signs and symptoms of hypersensitivity reactions. Instruct patients to stop taking Mesalamine Rectal Suppository and report to their healthcare provider if they experience new or worsening symptoms Acute Intolerance Syndrome (cramping, abdominal pain, bloody diarrhea, fever, headache, and rash) or other symptoms suggestive of mesalamine-induced hypersensitivity [see Warnings and Precautions (5.2, 5.3)].Hepatic Failure
Inform patients with known liver disease of the signs and symptoms of worsening liver function and advise them to report to their healthcare provider if they experience such signs or symptoms [see Warnings and Precautions (5.4)].Blood Disorders
Inform elderly patients and those taking azathioprine or 6-mercaptopurine of the risk for blood disorders and the need for periodic monitoring of complete blood cell counts and platelet counts while on therapy. Advise patients to complete all blood tests ordered by their healthcare provider [see Drug Interactions (7.2), Use in Specific Populations (8.5)].Manufactured for:
Amring Pharmaceuticals Inc.
Berwyn, PA 19312

Manufactured by:
Confab Laboratories Inc.
St-Hubert, Canada J3Y 3X3For more information, call 1-844-Amring1 (1-844-267-4641)
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
Mesalamine (me sal’ a meen) Suppositories, for rectal useWhat is Mesalamine Rectal Suppository?
Mesalamine Rectal Suppository is a prescription medicine used to treat adults with active ulcerative proctitis (ulcerative rectal colitis).
It is not known if Mesalamine Rectal Suppository is safe and effective in children.Do not use Mesalamine Rectal Suppository if you are:
- allergic to medicines that contain salicylates, including aspirin.
- allergic to mesalamine or any of the ingredients in Mesalamine Rectal Suppository. See the end of this Patient Information leaflet for a complete list of ingredients in Mesalamine Rectal Suppository.
Before using Mesalamine Rectal Suppository, tell your doctor if you:
- have a history of allergic reaction to the medicine sulfasalazine (Azulfidine).
- have kidney problems.
- have ever had inflammation of the sac around your heart (pericarditis).
- have liver problems.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if Mesalamine Rectal Suppository can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Mesalamine Rectal Suppository can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you use Mesalamine Rectal Suppository.
Using Mesalamine Rectal Suppository with certain other medicines may affect each other. Using Mesalamine Rectal Suppository with other medicines can cause serious side effects.
Especially tell your doctor if you take nonsteroidal anti-inflammatory drugs (NSAIDS), or medicines that contain azathioprine or 6-mercaptopurine. Taking Mesalamine Rectal Suppository with NSAIDS may cause kidney problems. Taking Mesalamine Rectal Suppository with azathioprine or 6-mercaptopurine may cause blood problems. You doctor may do certain tests during treatment with Mesalamine Rectal Suppository.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.How should I use Mesalamine Rectal Suppository? - Use Mesalamine Rectal Suppository exactly as prescribed by your doctor. Your doctor will tell you how long to continue using Mesalamine Rectal Suppository.
- Mesalamine Rectal Suppository comes as a suppository that you insert into your rectum.
- Do not cut or break the suppository.
- Use Mesalamine Rectal Suppository 1 time each day at bedtime, for 3 to 6 weeks. It is not known if Mesalamine Rectal Suppository is safe and effective for use for longer than 6 weeks.
- After you insert Mesalamine Rectal Suppository in your rectum, try to keep (retain) the suppository in your rectum for 1 to 3 hours or longer if possible.
- If you miss a dose of Mesalamine Rectal Suppository, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take the next dose at your regular time. Do not take 2 doses at the same time.
- Mesalamine Rectal Suppository can stain surfaces including clothing and other fabrics, flooring, painted surfaces, marble, granite, vinyl and enamel. Keep Mesalamine Rectal Suppository away from these surfaces to prevent staining.
What are the possible side effects of Mesalamine Rectal Suppository?
Mesalamine Rectal Suppository may cause serious side effects, including:- Kidney problems. Your doctor will do certain tests before you start using Mesalamine Rectal Suppository and during your treatment with Mesalamine Rectal Suppository.
- Acute Intolerance Syndrome or Other Allergic Reactions. Some people who use Mesalamine Rectal Suppository can have allergic type reactions, including “Acute Intolerance Syndrome.” Other allergic reactions can cause heart problems including an inflammation of the sac around the heart (pericarditis), blood problems, and problems with other organs in the body including the kidneys, liver and lungs. These problems usually happen in people who have had an allergic reaction to medicines containing sulfasalazine. Stop using Mesalamine Rectal Suppository and tell your doctor right away if you get any of these symptoms:
o cramps o fever o stomach (abdominal) pain o headache o bloody diarrhea o rash o chest pain o shortness of breath o decrease in the amount of urine o fatigue - Liver problems. This can happen in people who have a history of liver problems and have taken other medicines that contain mesalamine. Tell your doctor right away if you get any of these symptoms while using Mesalamine Rectal Suppository:
o yellowing of your eyes o flu-like symptoms o itchy skin o nausea or vomiting o feeling very tired The most common side effects of Mesalamine Rectal Suppository include: o dizziness o rectal pain o acne o fever o inflammation of the large intestine (colitis) o rash These are not all of the possible side effects of Mesalamine Rectal Suppository.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store Mesalamine Rectal Suppository?
- Store Mesalamine Rectal Suppository at room temperature below 77°F (25°C).
- Mesalamine Rectal Suppository may be refrigerated.
- Keep Mesalamine Rectal Suppository away from direct heat, light, or humidity.
General information about the safe and effective use of Mesalamine Rectal Suppository.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Mesalamine Rectal Suppository for a condition for which it was not prescribed. Do not give Mesalamine Rectal Suppository to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about Mesalamine Rectal Suppository that is written for health professionals.What are the ingredients in Mesalamine Rectal Suppository?
Active ingredients: mesalamine
Inactive ingredients: Hard Fat base
Manufactured for: Amring Pharmaceuticals Inc. Berwyn, PA 19312
Manufactured by: Confab Laboratories Inc. St-Hubert, Canada J3Y 3X3
Origin Canada
For more information, call 1-844-Amring1 (1-844-267-4641).This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 06/2019
301944-02
- PRINCIPAL DISPLAY PANEL – 1000 mg
-
INGREDIENTS AND APPEARANCE
MESALAMINE RECTAL
mesalamine suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69918-560 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 1000 mg Inactive Ingredients Ingredient Name Strength FAT, HARD (UNII: 8334LX7S21) Product Characteristics Color WHITE (white to beige) Score Shape BULLET (torpedo shaped suppository) Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69918-560-30 30 in 1 BOX; Type 0: Not a Combination Product 06/24/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208362 06/24/2019 Labeler - Amring Pharmaceuticals Inc. (079843051) Establishment Name Address ID/FEI Business Operations Confab Laboratories Inc. 241754217 analysis(69918-560) , manufacture(69918-560) , label(69918-560) , pack(69918-560)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.