4133 First Aid Kit by Honeywell Safety Products USA, INC 4133 FIRST AID KIT kit
4133 First Aid Kit by
Drug Labeling and Warnings
4133 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
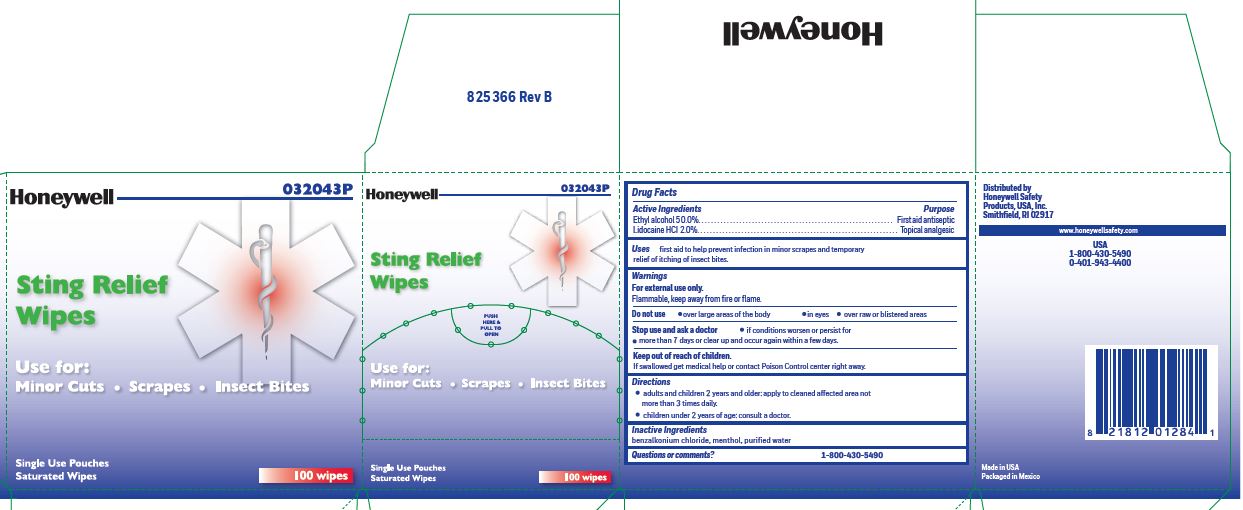
- Sting Relief Active ingredient
- Sting Relief Purpose
- Sting Relief Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting relief Questions or Comments
- Eyesaline Active ingredient
- Eyesaline Purpose
- Eyesaline Uses
-
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyesaline Directions
- Eyesaline Inactive ingredients
- Eyesaline Questions
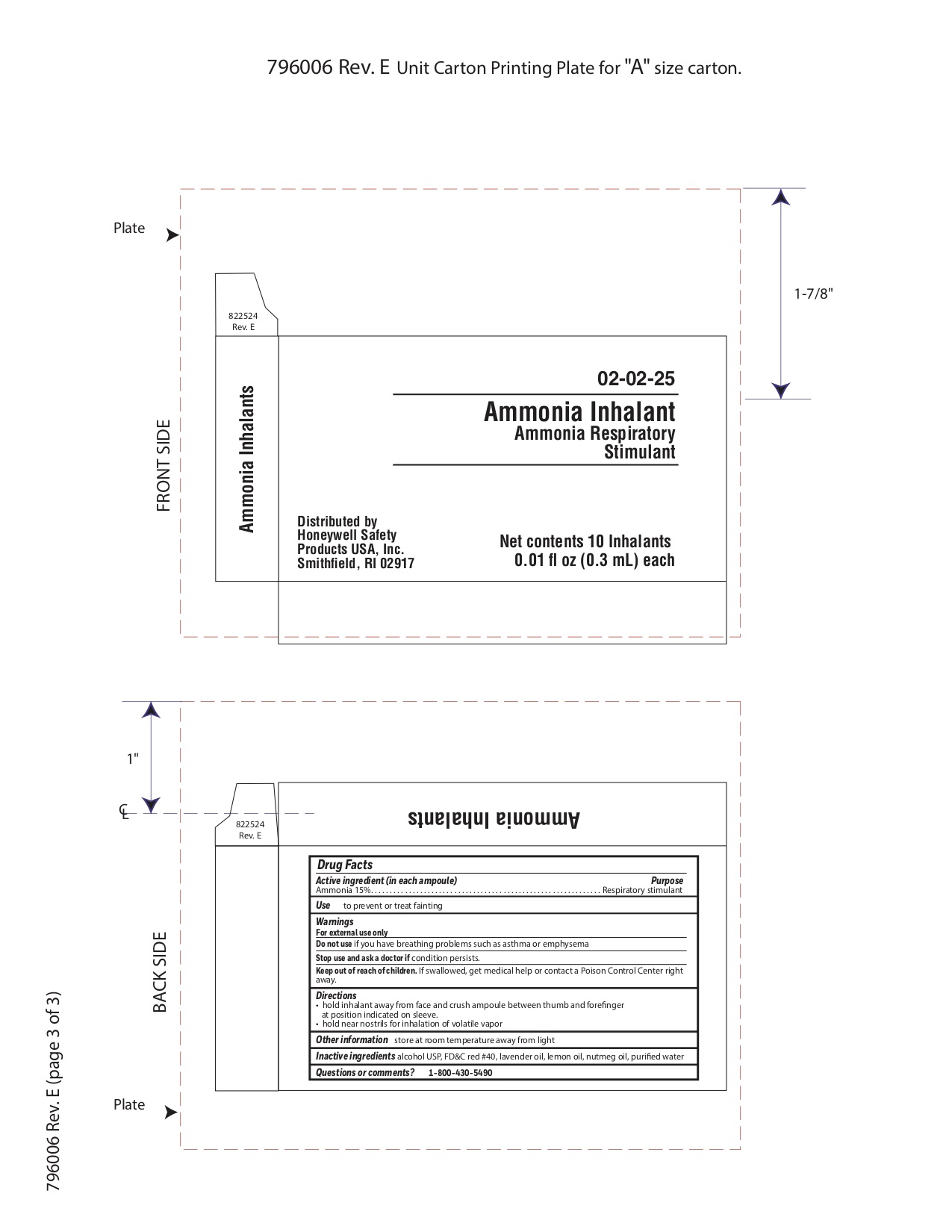
- Ammonia Inhalent Active ingredient
- Ammonia Inhalent Purpose
- Ammonia Inhalent Uses
- Ammonia Inhalent Warnings
- Ammonia Inhalent Directions
- Ammonia Inhalent Other information
- Ammonia Inhalent Other information
- Ammonia Inhalent Inactive ingredients
- Ammonia Inhalent Questions or Comments
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn Jel Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
- Povidone Iodine Swab Active ingredient
- Povidone Iodine Swab Purpose
- Povidone Iodine Swab Uses
- Povidone Iodine Swab Warnings
- Povidone Iodine Swab Directions
- Povidone Iodine Swab Other information
- Povidone Iodine Swab Inactive ingredients
- Povidone Iodine Swab Questions and comments
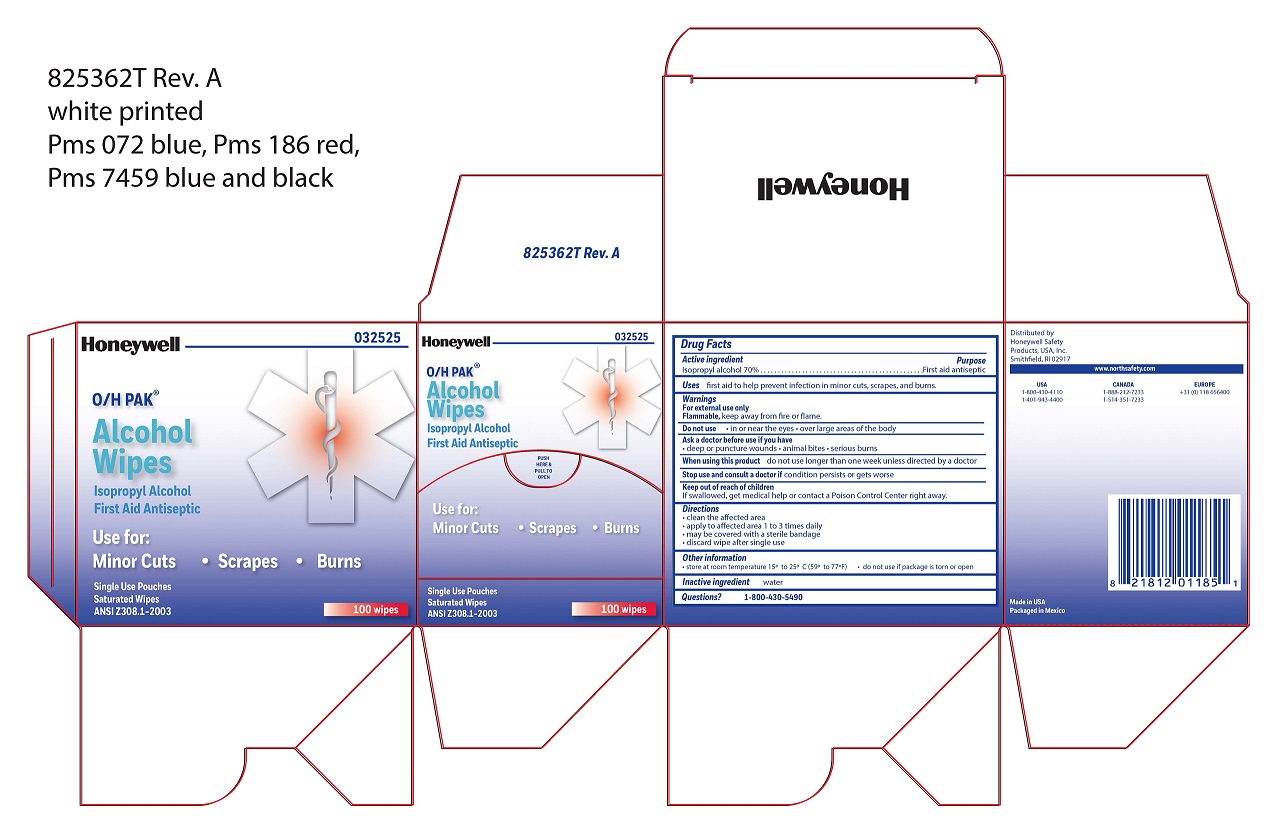
- Alcohol Wipe Active ingredient
- Alcohol Wipe Purpose
- Alcohol Wipe Uses
- Alcohol Wipe Warnings
- Alcohol Wipe Directions
- Alcohol Wipe Other information
- Alcohol Wipe Inactive ingredient
- Alcohol Wipe Questions
-
4133
SF00004171 Kit Contents
1 KNUCKLE BAND 8 PER
1 AMMONIA INHALANTS 10 PER
1 GAUZE BANDAGE, 4" X 6 YD
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 ADHESIVE TPE 1"X2-1/2 YD 2 PER
2 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 1 OZ EYE WASH W/PADS & STRIPS
1 BURN JEL 1/8 OZ, 6 PER
1 PVP IODINE WIPES 10 PER
1 STING RELIEF WIPES 10 PER BOX
1 MICROSHIELD W/VNL GLV/ALCL
LBL STOCK 6-3/8"X4"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 16 UN (VERTICAL)
1 LABL INSTR FA REV A
- Sting Relief Principal Display Panel
- Eyesaline Principal Display Panel
- Ammonia inhalent Principal Display Panel
- Burn Jel Principal Display Panel
- Povidone Iodine Swab Principal Display Panel
- Alcohol Wipe Principal Display Panel
- 4133 Kit Label SF00004171
-
INGREDIENTS AND APPEARANCE
4133 FIRST AID KIT
4133 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4133 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4133-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 POUCH 4 mL Part 2 1 BOTTLE 30 mL Part 3 10 AMPULE 3 mL Part 4 4 POUCH 1.6 mL Part 5 10 POUCH 3 mL Part 6 6 PACKET 21 g Part 1 of 6 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Part 2 of 6 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 3 of 6 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC: 0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 4 of 6 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 5 of 6 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 6 of 6 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) DIPROPYLENE GLYCOL (UNII: E107L85C40) TEA TREE OIL (UNII: VIF565UC2G) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations James Alexander 040756421 manufacture(0498-3334) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4133) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0203) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0143) Establishment Name Address ID/FEI Business Operations Sion Biotext Medical 532775194 manufacture(0498-0121) Establishment Name Address ID/FEI Business Operations Safetec of America Inc 874965262 manufacture(0498-0733)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.