Cardinal Health Lubricant Eye Drops Drug Facts
leader lubricant eye by
Drug Labeling and Warnings
leader lubricant eye by is a Otc medication manufactured, distributed, or labeled by Cardinal Health. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEADER LUBRICANT EYE- polyethylene glycol, propylene glycol solution, gel forming / drops
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
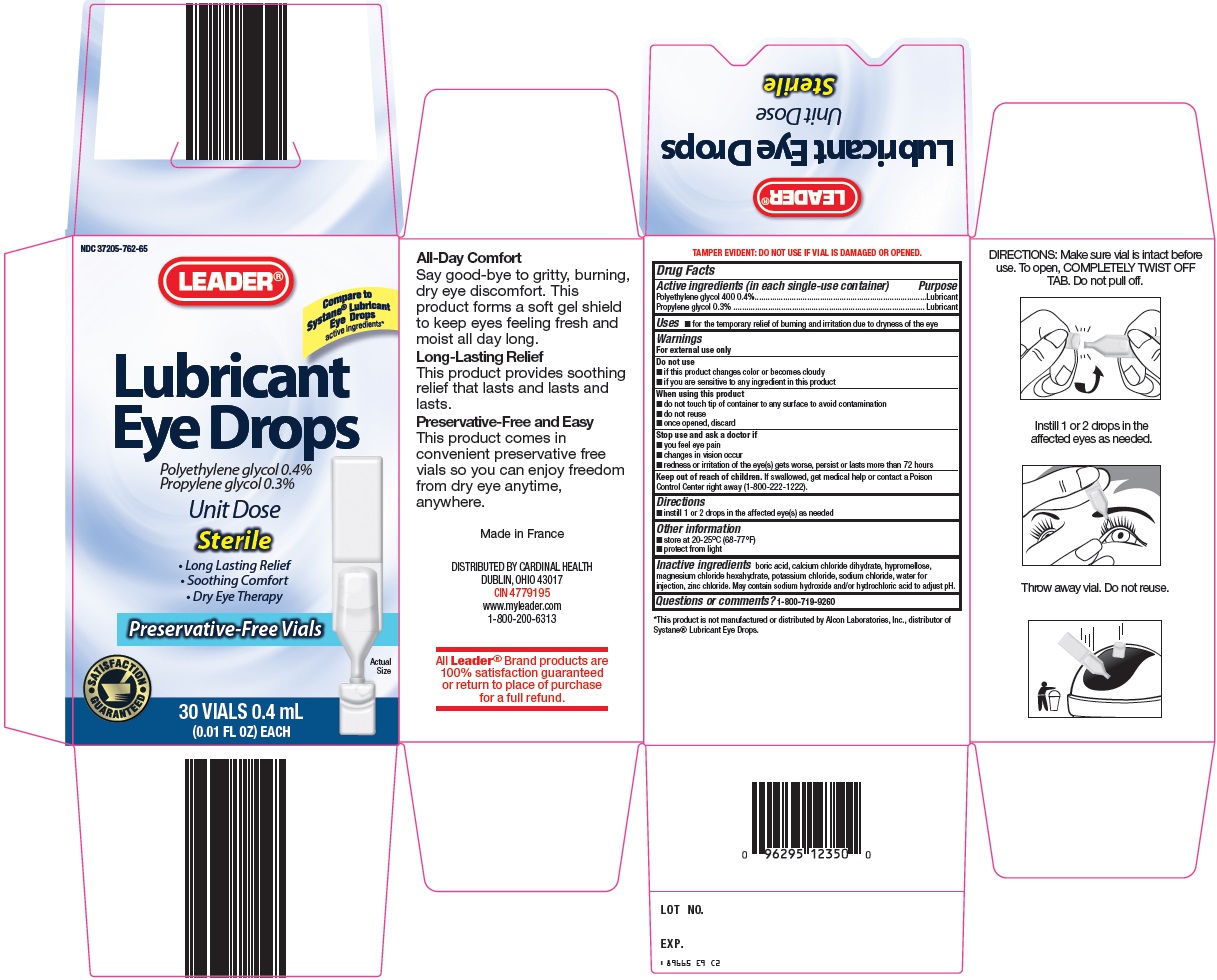
Cardinal Health Lubricant Eye Drops Drug Facts
Active ingredients (in each single-use container)
Polyethylene glycol 400 0.4%
Propylene glycol 0.3%
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- do not reuse
- once opened, discard
Inactive ingredients
boric acid, calcium chloride dihydrate, hypromellose, magnesium chloride hexahydrate, potassium chloride, sodium chloride, water for injection, zinc chloride. May contain sodium hydroxide and/or hydrochloric acid to adjust pH.
Package/Label Principal Display Panel
Compare to Systane® Lubricant Eye Drops active ingredients
Lubricant Eye Drops
Polyethylene glycol 0.4%
Propylene glycol 0.3%
Unit Dose
Sterile
Long Lasting Relief
Soothing Comfort
Dry Eye Therapy
Preservative-Free Vials
Actual Size
30 VIALS 0.4 mL
(0.01 FL OZ) EACH
| LEADER LUBRICANT EYE
polyethylene glycol, propylene glycol solution, gel forming / drops |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Cardinal Health (097537435) |