4129 First Aid Kit by Honeywell Safety Products USA, INC 4129 FIRST AID KIT kit
4129 First Aid Kit by
Drug Labeling and Warnings
4129 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
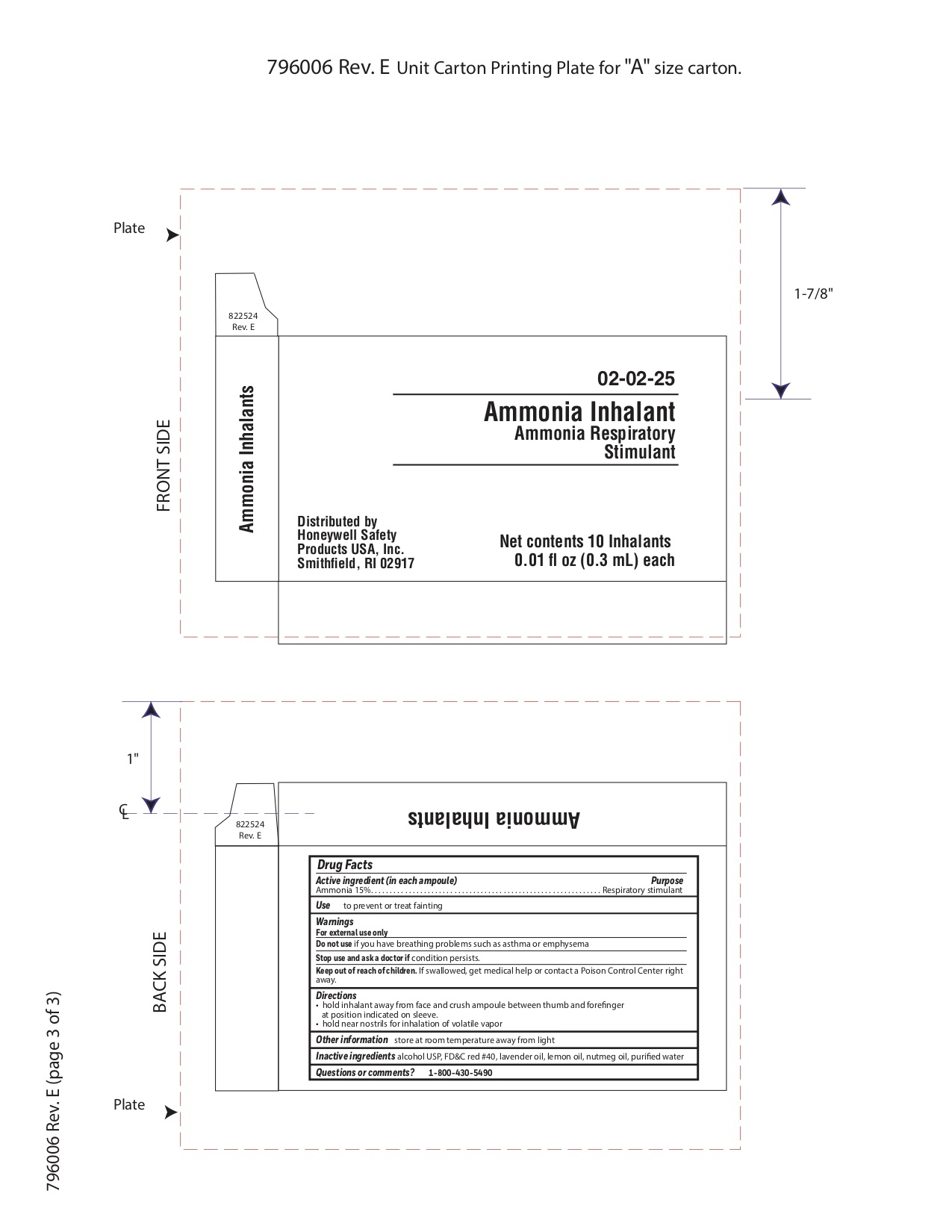
- Ammonia Active ingredient
- Ammonia Purpose
- Ammonia Uses
- Ammonia Warnings
- Ammonia Directions
- Ammonia Other information
- Ammonia Inactive ingredient
- Ammonia Questions or Comments?
- Pyrocaine Active ingredient
- Pyrocaine Purpose
-
Pyrocaine
Uses
For the temporary relief of pain and itching, and to help protect against skin infection in:
- minor burns
- minor skin irritations
- minor cuts and scrapes
- insect bites
- sunburns
- Pyrocaine Warnings
- Pyrocaine Directions
- Pyrocaine Other information
- Pyrocaine Inactive ingredients
- PVP Active ingredient
- PVP Purpose
- PVP Uses
- PVP Warnings
- PVP Directions
- PVP Other information
- PVP Inactive ingredients
- PVP Questions
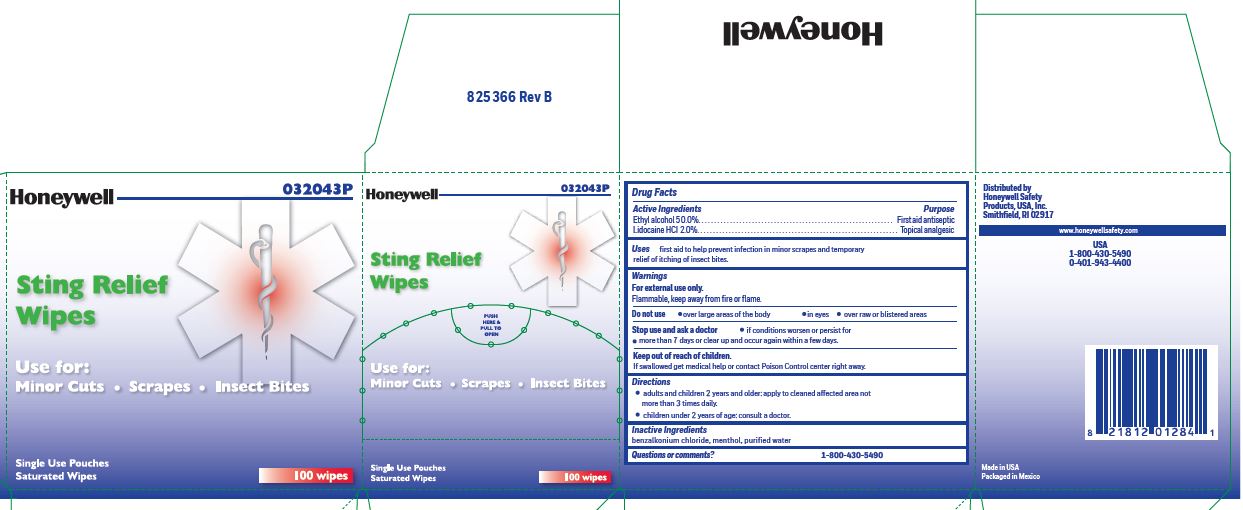
- Sting Relief Active ingredient (in each wipe)
- Sting Relief Purpose
- Sting Relef Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting Relief Questions or Comments?
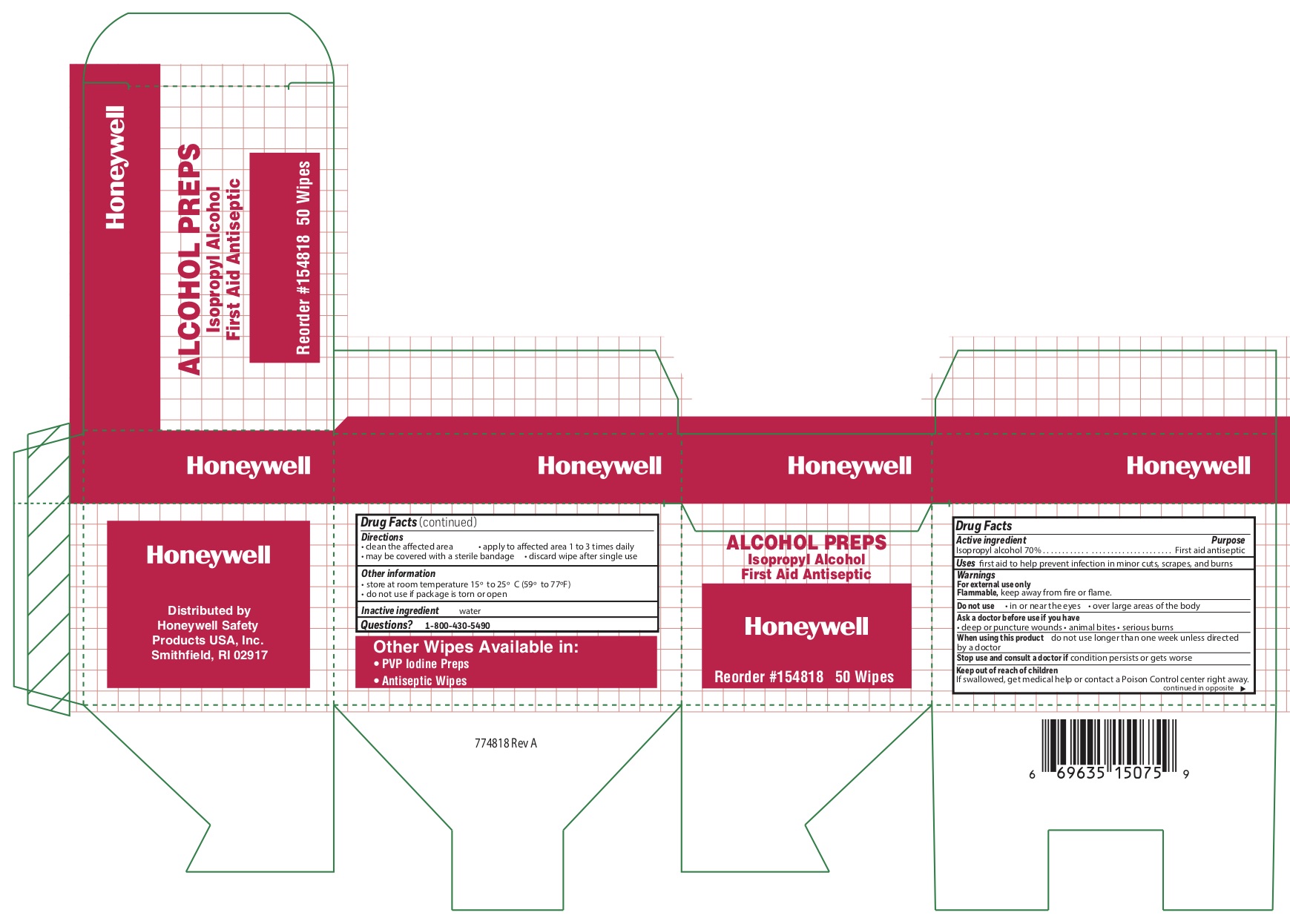
- alcohol Active ingredient
- Alcohol Purpose
- Alcohol Uses
- Alcohol Warnings
- Alcohol Directions
- Alcohol Other information
- Alcohol Inactive ingredient
- Alcojol Questions
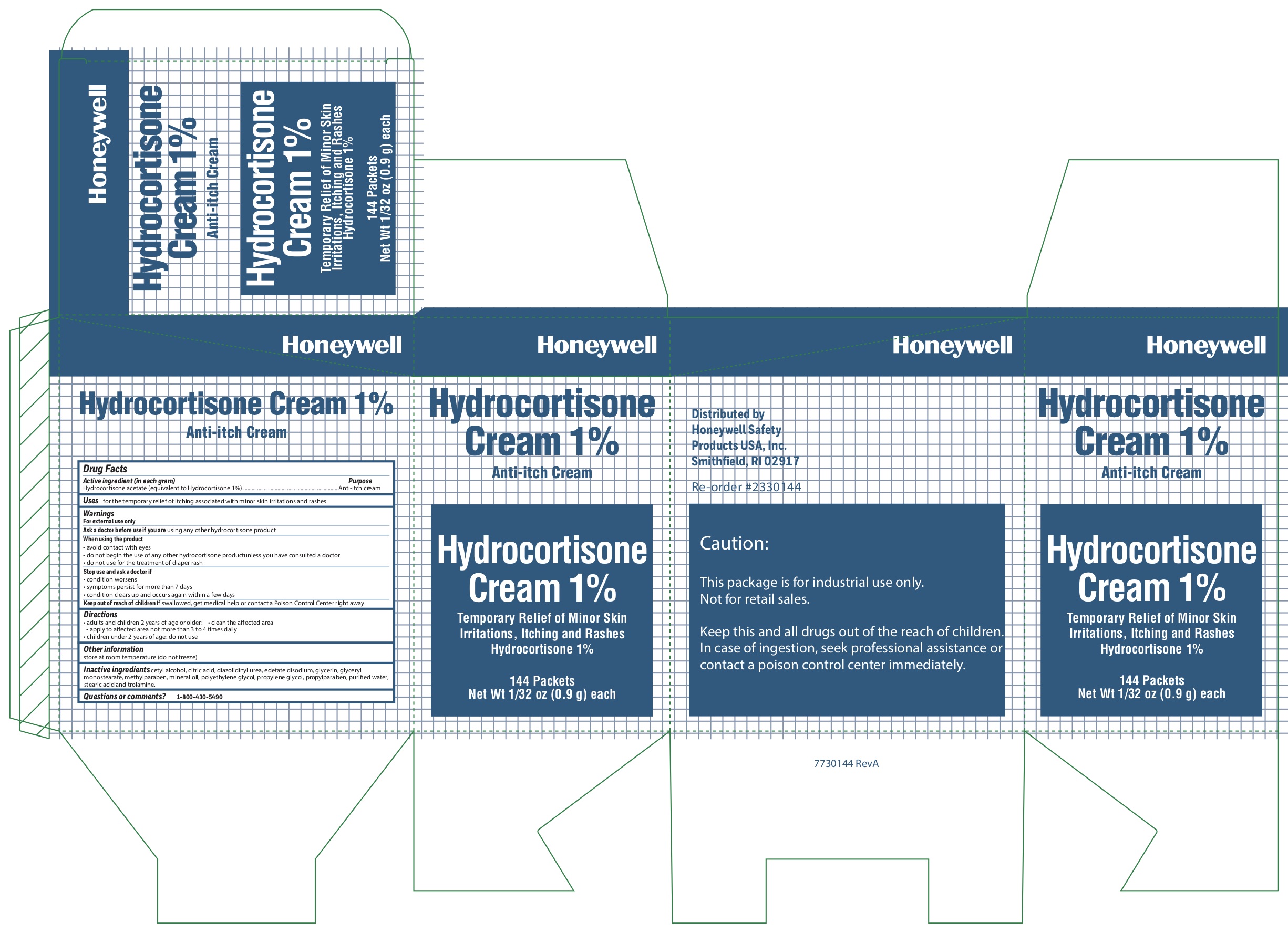
- Hydrocortisone Active ingredient (in each gram)
- Hydrocortisone Purpose
- Hydrocortisone Uses
-
Hydrocortisone
Warnings
For external use only
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
- Hydrocortisone Directions
- Hydrocortisone Other information
- Hydrocortisone Inactive ingredients
- Hydrocortisone Questions or comments?
- BZK Active ingredient
- BZK Purpose
- BZK Uses
-
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Directions
- BZK Other information
- BZK Inactive ingredient
- BZK Questions
-
4129
SF00004226 Kit Contents
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 TRIANGULAR BDG, NON-STERILE
1 INSTANT COLD PACK 4" X 6"
1 ALCOHOL PREP PADS 10P
1 HYDROCORTISON,1.O%,1/32 OZ,10P
1 RESPONSE KIT BLOODBORNE PATHOG
1 SCISSOR BDGE 4" RED PLS HDL
1 BANDAGE COMP 2" W/TELFA PAD 4
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
1 KIT STL 24 UN WHITE 01
1 PVP IODINE SWABS 10
1 STING Relief SWAB 10
1 PYRO-CAINE AERO 2/BX
1 RED BIO BAGS 2/BX
1 FACE MASK/EYE SHIELD
1 LIQD TRTMNT SYS 1 EA
1 DISP. TOWEL/WIPES 2EA
1 IMPERVIOUS GOWN 1 EA
- Ammonia Principal Display Panel
- Pyrocaine Principal Display Panel
- PVP Principal Display Panel
- Sting Relief Principal Display Panel
- Alcohol Principal Display Panel
- Hydrocortisone Principal Display Panel
- BZK Principal Display Panel
- 4129 Kit Label SF00004226
-
INGREDIENTS AND APPEARANCE
4129 FIRST AID KIT
4129 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4129 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4129-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 PACKET 9 g Part 2 10 AMPULE 3 mL Part 3 2 CAN 28.4 g Part 4 10 POUCH 4 mL Part 5 10 POUCH 3 mL Part 6 4 PACKET 5.6 mL Part 7 10 POUCH 4 mL Part 8 10 PACKET 9 g Part 1 of 8 HYDROCORTISONE
anti-itch creamProduct Information Item Code (Source) NDC: 0498-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) CETYL ALCOHOL (UNII: 936JST6JCN) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/06/2013 Part 2 of 8 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC: 0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 8 PYROCAINE BURN
benzocaine, benzethonium chloride aerosol, sprayProduct Information Item Code (Source) NDC: 0498-0011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 g BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength 1,1,3-TRI(3-TERT-BUTYL-4-HYDROXY-6-METHYLPHENYL)BUTANE (UNII: BF6E9O0XJN) ISOBUTANE (UNII: BXR49TP611) 1,1,3-TRIS(2-CHLOROETHOXY)PROPANE (UNII: 4FEX9N888E) DIPROPYLENE GLYCOL (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0011-77 14.2 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2018 Part 4 of 8 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MENTHOL (UNII: L7T10EIP3A) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0733-00 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Part 5 of 8 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 6 of 8 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/21/2017 Part 7 of 8 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 8 of 8 HYDROCORTISONE
anti-itch cream ointmentProduct Information Item Code (Source) NDC: 0498-0800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) CETYL ALCOHOL (UNII: 936JST6JCN) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0800-34 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/06/2013 10/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations James Alexander 040756421 manufacture(0498-3334) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4129) Establishment Name Address ID/FEI Business Operations Dixon Investments 115315822 manufacture(0498-0011) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0800, 0498-0801) Establishment Name Address ID/FEI Business Operations Chanzhou Maokang Medical 421317073 manufacture(0498-0143, 0498-0501) Establishment Name Address ID/FEI Business Operations Sion Medical Biotext 532775194 manufacture(0498-0121) Establishment Name Address ID/FEI Business Operations Safetec of America Inc 874965262 manufacture(0498-0733)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.