4327 First Aid Kit by Honeywell Safety Products USA, INC 4327 FIRST AID KIT kit
4327 First Aid Kit by
Drug Labeling and Warnings
4327 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
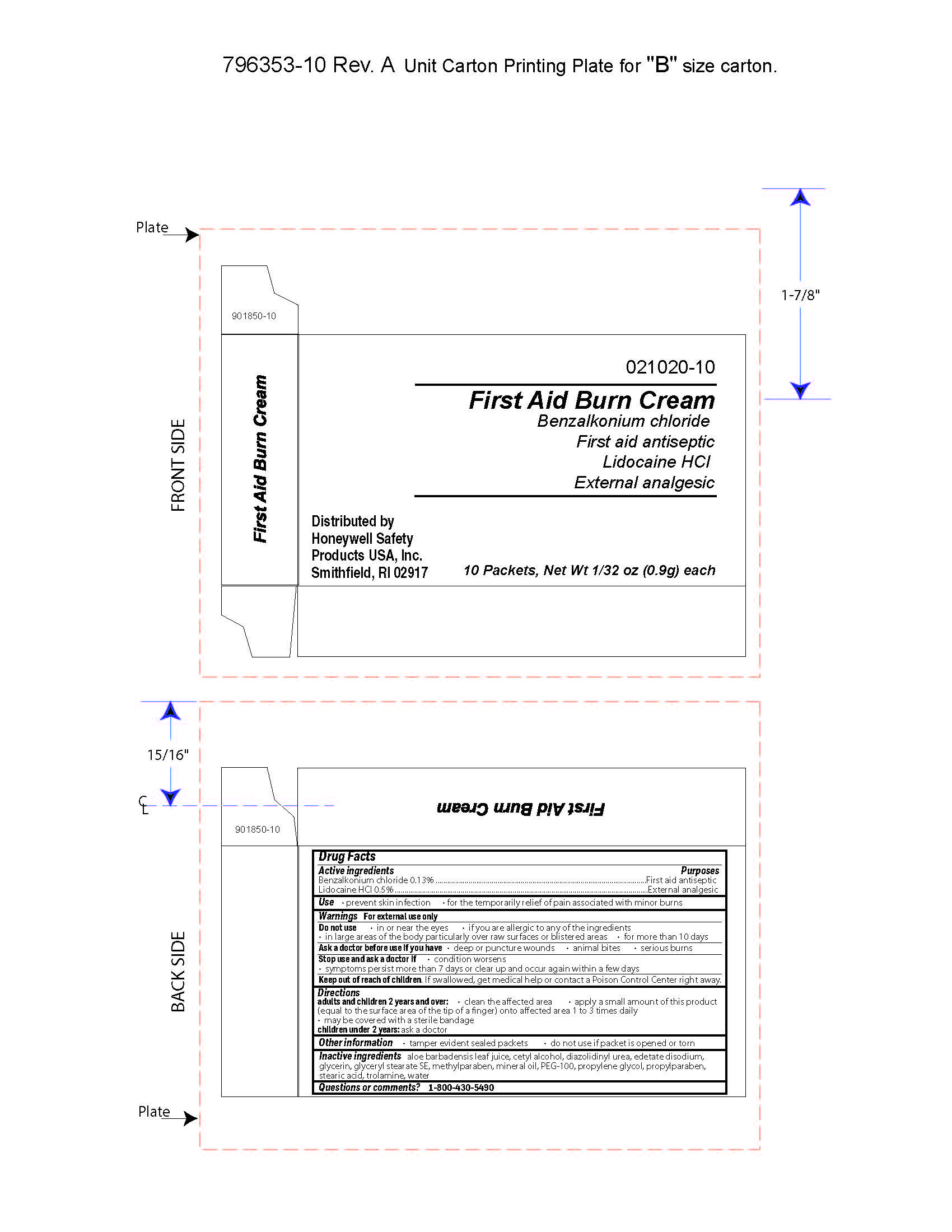
- First Aid Burn Cream Active ingredient
- First Aid Burn Cream Purpose
- First Aid Burn Cream Uses
- First Aid Burn Cream Warnings
- First Aid Burn Cream Directions
- First Aid Burn Cream Other information
- First Aid Burn Cream Inactive ingredients
- First Aid Burn Cream Questions
- BZK Antiseptic Wipe Active ingredient
- BZK Purpose
- BZK Uses
-
BZK
Warnings
For external use only
BZK
Do not use- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Directions
- BZK Other information
- BZK Inactive ingredients
- BZK Questions
- Aypanal Active igredient
- Aypanal Purpose
- Aypanal Uses
-
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take: more than 4,000 mg in 24 hours, which is the maximum daily amount - child takes
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
skin reddening
blisters
rash -
Aypanal
Directions
- do not take more than directed (see overdose warning) adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than directed (see overdose warning)
adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
- children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- children under 6 years
- consult a doctor
- Aypanal Other information
- Aypanal Inactive ingredients
- Aypanal Questions or Comments?
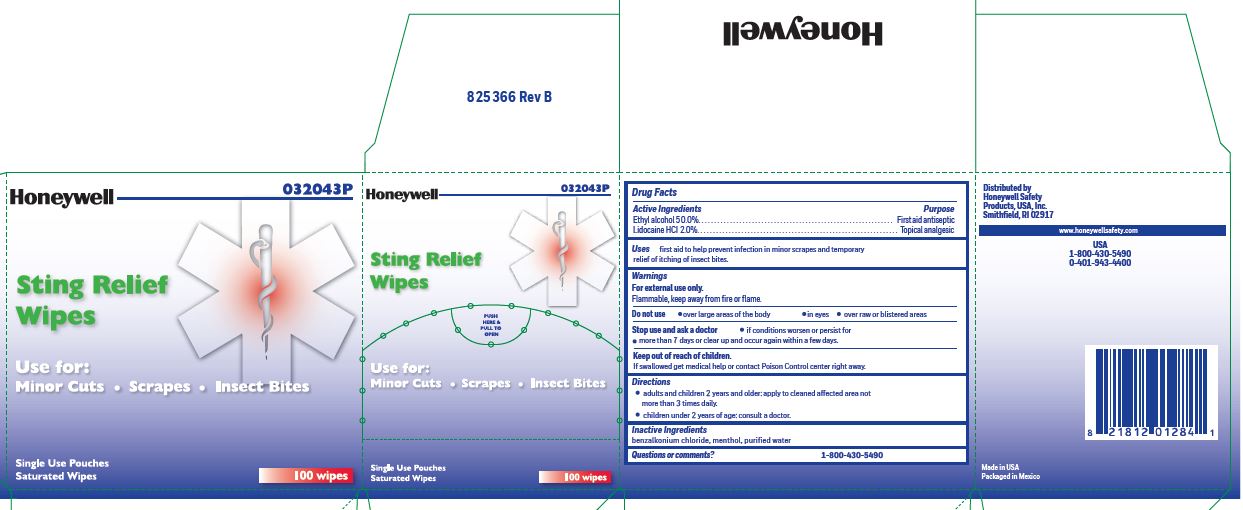
- Sting Relief Active ingredient
- Sting Relief Purpose
- Sting Relief Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting relief Questions or Comments
- Neomycin Antibiotic Ointment Active ingredient
- Neomycin Antibiotic Ointment Purpose
- Neomycin Antibiotic Ointment Uses
- Neomycin Antibiotic Ointment Warnings
- Neomycin Antibiotic Ointment Directions
- Neomycin Antibiotic Ointment Other information
- Neomycin Antibiotic Ointment Inactive ingredient
- Neomycin Antibiotic Ointment Questions
- Eyewash Active ingredient
- Eyewash Purpose
- Eyewash Uses
-
Eyeash
Warnings
For external use only- Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyewash Directions
- Eyewash Inactive ingredients
- Eyewash Questions?
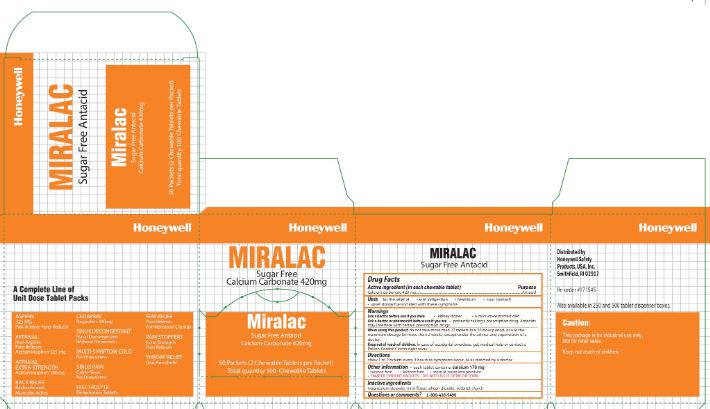
- Miralac Active ingredient (In Each Chewable Tablet)
- Miralac Purpose
- Miralac Uses
-
Miralac
Warnings
Ask a doctor before use if you are
- presently taking a prescription drug. Antacids may interfere with certain prescription drugs
- Miralac Directions
- Miralac Other information
- Miralac Inactive ingredients
- Miralac Questions
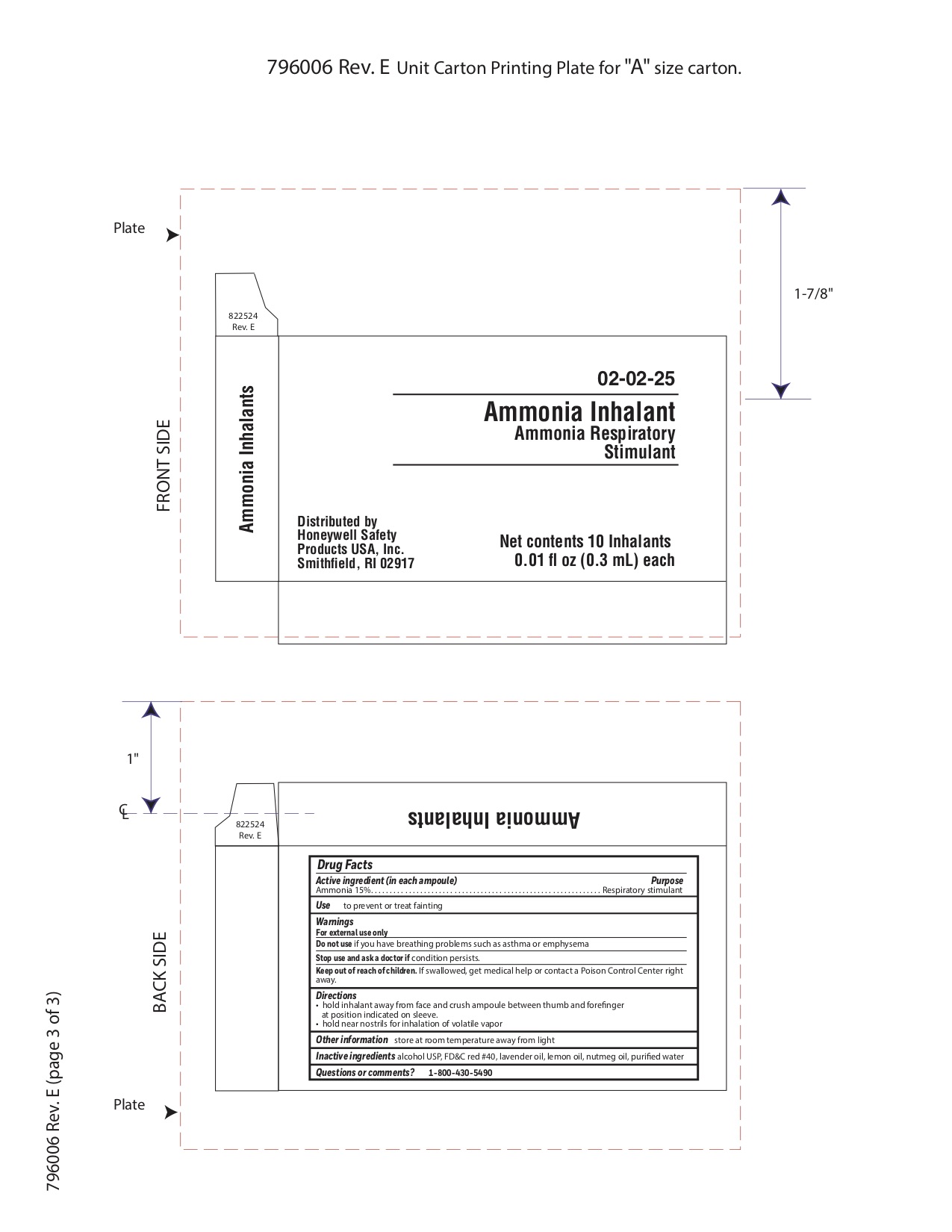
- Ammonia Active ingredient
- Ammonia Purpose
- Ammonia Uses
- Ammonia Warnings
- Ammonia Directions
- Ammonia Other information
- Ammonia Inactive ingredients
- Ammonia Questions or Comments?
-
4327
Z019738-0025L Kit Contents
1 1X3 PLASTIC 100/BOX
1 TWEEZER PLASTICS 4"
1 FIRST AID GUIDE ASHI
1 CO-FLEX BANDAGE 2"X 5YDS TAN
1 CPR FILTERSHIELD 77-100
1 BAGGED COMP MISC
1 212 4 OZ TUBE SKIN COND, 1 EA ID P
1 1 OZ, BUFF EYEWASH
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 LBL CONTS 6 3/4"X3 1/2" ID B
2 PR LRG NITRILE GLVES ZIP BAG
1 TAPE ADHESIVE 1/2 X 2.5 125133
1 KIT, PP 16 UNIT FA
1 LABEL 16U CVR NORTH OFFICE
1 COLD PACK UNIT 4"X6" BULK
2 EYE PADS STD OVAL STERILE
2 GAUZE PADS 3"X3" 12PLY
6 MIRALAC BULK 2/PK
2 AMMONIA INHALANT, BULK
- First Aid Burn Cream Principal Display Panel
- BZK Principal Display Panel
- Aypanal Principal Display Panel
- Sting Relief Principal Display Panel
- Neomycin Antibiotic Ointment Principal Display Panel
- Eyewash Principal Display Pane
- Miralac Principal Display Panel
- Ammonia Principal Display Panel
- 4327 Kit Lael Z019738-0025L
-
INGREDIENTS AND APPEARANCE
4327 FIRST AID KIT
4327 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4327 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4327-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 3 PACKET 6 Part 2 6 POUCH 2.4 mL Part 3 10 PACKET 9 g Part 4 10 PACKET 9 g Part 5 10 PACKET 14 mL Part 6 1 BOTTLE 30 mL Part 7 2 AMPULE 0.6 mL Part 8 6 PACKET 12 Part 1 of 8 AYPANAL NON-ASPIRIN
acetaminophen tabletProduct Information Item Code (Source) NDC: 0498-2001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code circle;U Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-2001-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 04/10/2012 Part 2 of 8 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Part 3 of 8 FIRST AID BURN
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Item Code (Source) NDC: 0498-0903 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) METHYLPARABEN (UNII: A2I8C7HI9T) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) LIGHT MINERAL OIL (UNII: N6K5787QVP) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 12/20/2017 Part 4 of 8 NEOMYCIN
antibiotic ointmentProduct Information Item Code (Source) NDC: 0498-0730 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0730-01 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 03/31/2010 Part 5 of 8 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/21/2017 Part 6 of 8 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/15/2018 Part 7 of 8 AMMONIA INHALENT
ammonia inhalent inhalantProduct Information Item Code (Source) NDC: 0498-3334 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMMONIA (UNII: 5138Q19F1X) (AMMONIA - UNII:5138Q19F1X) AMMONIA 0.045 g in 0.3 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-3334-00 0.3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 8 of 8 MIRALAC
calcium carbonate tabletProduct Information Item Code (Source) NDC: 0498-0303 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (white) Score 2 pieces Shape ROUND Size 11mm Flavor MINT (Mint Flavor) Imprint Code FR8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 02/22/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations James Alexander 040756421 manufacture(0498-3334) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4327) Establishment Name Address ID/FEI Business Operations Ultra Seal Corporation 085752004 manufacture(0498-2001, 0498-0303) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0903, 0498-0730) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0501) Establishment Name Address ID/FEI Business Operations Safetec of America Inc 874965262 manufacture(0498-0733)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.