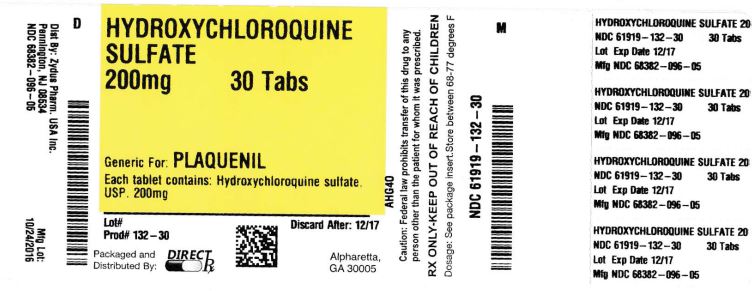
HYDROXYCHLOROQUINE SULFATE
HYDROXYCHLOROQUINE SULFATE by
Drug Labeling and Warnings
HYDROXYCHLOROQUINE SULFATE by is a Prescription medication manufactured, distributed, or labeled by DIRECT RX. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HYDROXYCHLOROQUINE SULFATE- hydroxychloroquine sulfate tablet, film coated
DIRECT RX
----------
HYDROXYCHLOROQUINE SULFATE
BOXED WARNING SECTION
WARNING
PHYSICIANS SHOULD COMPLETELY FAMILIARIZE THEMSELVES WITH THE COMPLETE CONTENTS OF THIS LEAFLET BEFORE PRESCRIBING HYDROXYCHLOROQUINE.
DESCRIPTION SECTION
Hydroxychloroquine sulfate is an odorless, white or practically white crystalline powder, freely soluble in water; practically insoluble in alcohol, in chloroform, and in ether. Chemically the drug is 2-[[4-[(7-Chloro-4-quinolyl) amino] pentyl] ethylamino] ethanol sulfate (1:1).
Each hydroxychloroquine sulfate tablet intended for oral administration contains 200 mg of hydroxychloroquine sulfate equivalent to 155 mg base. In addition, each tablet contains the following inactive ingredients: dibasic calcium phosphate dihydrate, magnesium stearate, pregelatinized starch, polyethylene glycol, polyvinyl alcohol, starch, talc and titanium dioxide.
CLINICAL PHARMACOLOGY SECTION
The drug possesses antimalarial actions and also exerts a beneficial effect in lupus erythematosus (chronic discoid or systemic) and acute or chronic rheumatoid arthritis. The precise mechanism of action is not known.
INDICATIONS & USAGE SECTION
Hydroxychloroquine sulfate tablets are indicated for the suppressive treatment and treatment of acute attacks of malaria due to Plasmodium vivax, P. malariae, P. ovale, and susceptible strains of P. falciparum. It is also indicated for the treatment of discoid and systemic lupus erythematosus, and rheumatoid arthritis.
CONTRAINDICATIONS SECTION
Use of this drug is contraindicated (1) in the presence of retinal or visual field changes attributable to any 4-aminoquinoline compound, (2) in patients with known hypersensitivity to 4-aminoquinoline compounds, and (3) for long-term therapy in children
WARNINGS SECTION
General
Hydroxychloroquine sulfate tablets are not effective against chloroquine-resistant strains of P. falciparum.
Children are especially sensitive to the 4-aminoquinoline compounds. A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g in one 3-year-old child). Patients should be strongly warned to keep these drugs out of the reach of children.
Use of hydroxychloroquine sulfate tablets in patients with psoriasis may precipitate a severe attack of psoriasis. When used in patients with porphyria the condition may be exacerbated. The preparation should not be used in these conditions unless in the judgment of the physician the benefit to the patient outweighs the possible hazard.
Usage in Pregnancy
Usage of this drug during pregnancy should be avoided except in the suppression or treatment of malaria when in the judgment of the physician the benefit outweighs the possible hazard. It should be noted that radioactively-tagged chloroquine administered intravenously to pregnant, pigmented CBA mice passed rapidly across the placenta. It accumulated selectively in the melanin structures of the fetal eyes and was retained in the ocular tissues for five months after the drug had been eliminated from the rest of the body.
PRECAUTIONS SECTION
General
Antimalarial compounds should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Periodic blood cell counts should be made if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuation of the drug should be considered. The drug should be administered with caution in patients having G-6-PD (glucose-6-phosphate dehydrogenase) deficiency.
OVERDOSAGE SECTION
The 4-aminoquinoline compounds are very rapidly and completely absorbed after ingestion, and in accidental overdosage, or rarely with lower doses in hypersensitive patients, toxic symptoms may occur within 30 minutes. These consist of headache, drowsiness, visual disturbances, cardiovascular collapse, and convulsions, followed by sudden and early respiratory and cardiac arrest. The electrocardiogram may reveal atrial standstill, nodal rhythm, prolonged intraventricular conduction time, and progressive bradycardia leading to ventricular fibrillation and/or arrest. Treatment is symptomatic and must be prompt with immediate evacuation of the stomach by emesis (at home, before transportation to the hospital) or gastric lavage until the stomach is completely emptied. If finely powdered, activated charcoal is introduced by the stomach tube, after lavage, and within 30 minutes after ingestion of the tablets, it may inhibit further intestinal absorption of the drug. To be effective, the dose of activated charcoal should be at least five times the estimated dose of hydroxychloroquine ingested. Convulsions, if present, should be controlled before attempting gastric lavage. If due to cerebral stimulation, cautious administration of an ultrashort-acting barbiturate may be tried but, if due to anoxia, it should be corrected by oxygen administration, artificial respiration or, in shock with hypotension, by vasopressor therapy. Because of the importance of supporting respiration, tracheal intubation or tracheostomy, followed by gastric lavage, may also be necessary. Exchange transfusions have been used to reduce the level of 4-aminoquinoline drug in the blood.
A patient who survives the acute phase and is asymptomatic should be closely observed for at least six hours. Fluids may be forced, and sufficient ammonium chloride (8 g daily in divided doses for adults) may be administered for a few days to acidify the urine to help promote urinary excretion in cases of both overdosage and sensitivity.
HOW SUPPLIED SECTION
Hydroxychloroquine Sulfate Tablets, USP contain 200 mg of hydroxychloroquine sulfate, equivalent to 155 mg base, are white to off-white, capsule-shaped, biconvex, film-coated tablets debossed with "ZC38" on one side and plain on other side, and are supplied as follows:
NDC: 68382-096-01 in bottles of 100 tablets
NDC: 68382-096-05 in bottles of 500 tablets
NDC: 68382-096-30 in unit-dose blister cartons of 100 (10 x 10) unit dose tablets
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP/NF.
| HYDROXYCHLOROQUINE SULFATE
hydroxychloroquine sulfate tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - DIRECT RX (079254320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DIRECT RX | 079254320 | relabel(61919-132) , repack(61919-132) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.