XURIDEN- uridine triacetate granule
XURIDEN by
Drug Labeling and Warnings
XURIDEN by is a Prescription medication manufactured, distributed, or labeled by BTG International Inc, Almac Pharma Services, Almac Sciences, Butterworth Laboratories Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XURIDEN safely and effectively. See full prescribing information for XURIDEN.
XURIDEN® (uridine triacetate) oral granules
Initial U.S. Approval: 2015INDICATIONS AND USAGE
XURIDEN is a pyrimidine analog for uridine replacement indicated in adult and pediatric patients for the treatment of hereditary orotic aciduria. ( 1)
DOSAGE AND ADMINISTRATION
Recommended Dosage ( 2.1):
- The starting dosage is 60 mg/kg once daily; the dose may be increased to120 mg/kg (not to exceed 8 grams) once daily for insufficient efficacy.
- See the full prescribing information for 60 mg/kg and 120 mg/kg weight-based dosing tables.
Preparation and Administration ( 2.2)
- Measure the dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon, accurate to the fraction of the dose to be administered.
- Administer the dose with food (applesauce, pudding or yogurt) or in milk or infant formula. See full prescribing information for preparation and administration instructions.
DOSAGE FORMS AND STRENGTHS
Oral granules: 2 gram packets. ( 3)
CONTRAINDICATIONS
None ( 4)
ADVERSE REACTIONS
No adverse reactions were reported in clinical trials with XURIDEN in patients with hereditary orotic aciduria. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BTG International Inc. at (1-877-377-3784) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended starting dosage of oral XURIDEN is 60 mg/kg once daily. Increase the dosage of XURIDEN to 120 mg/kg (not to exceed 8 grams) once daily for insufficient efficacy, such as occurrence of one of the following:
- Levels of orotic acid in urine remain above normal or increase above the usual or expected range for the patient
- Laboratory values (e.g., red blood cell or white blood cell indices) affected by hereditary orotic aciduria show evidence of worsening
- Worsening of other signs or symptoms of the disease
The XURIDEN dose to be administered at the 60 mg/kg and 120 mg/kg dose levels by body-weight is presented in Tables 1 and 2.
A 2 gram packet of XURIDEN contains approximately ¾ teaspoon of XURIDEN. Therefore, in the tables below for patients requiring doses in multiples of 2 grams (¾ teaspoon) an entire packet(s) may be administered without weighing or measuring.
XURIDEN Daily Dose Based on Body Weight (kg)
Patient Weight Table 1: XURIDEN 60 mg/kg *Dose Level Kilograms Dose to be Administered Using a Scale (grams) Dose in Teaspoons - * total daily dose by weight category in the tables was rounded to achieve the approximate dose level
- † may use 1 entire 2 gram packet without weighing or measuring
- ‡ may use 2 entire 2 gram packets without weighing or measuring
- § may use 3 entire 2 gram packets without weighing or measuring
up to 5 0.4 ⅛ 6-10 0.4 to 0.6 ¼ 11-15 0.7 to 0.9 ½ 16-20 1 to 1.2 21-25 1.3 to 1.5 26-30 1.6 to 1.8 ¾ † 31-35 1.9 to 2.1 † 36-40 2.2 to 2.4 1 41-45 2.5 to 2.7 46-50 2.8 to 3 51-55 3.1 to 3.3 1 ¼ 56-60 3.4 to 3.6 61-65 3.7 to 3.9 ‡ 1 ½ ‡ 66-70 4 to 4.2 ‡ 71-75 4.3 to 4.5 Above 75 6 § 2 § Patient Weight Table 2: XURIDEN 120 mg/kg *Dose Level Kilograms Dose to be Administered Using a Scale (grams) Dose in Teaspoons - * total daily dose by weight category in the tables was rounded to achieve the approximate dose level
- † may use 2 entire 2 gram packets without weighing or measuring
- ‡ may use 3 entire 2 gram packets without weighing or measuring
- § may use 4 entire 2 gram packets without weighing or measuring
up to 5 0.8 ¼ 6-10 0.8 to 1.2 ½ 11-15 1.4 to 1.8 ¾ 16-20 2 to 2.4 1 21-25 2.6 to 3 26-30 3.2 to 3.6 1 ¼ 31-35 3.8 to 4.2 † 1 ½ † 36-40 4.4 to 4.8 1 ¾ 41-45 5 to 5.4 2 ‡ 46-50 5.6 to 6 51-55 6.2 to 6.6 2 ¼ 56-60 6.8 to 7.2 2 ½ 61-65 7.4 to 7.8 66-70 8 § 2 ¾ § 71-75 8 § Above 75 8 § 2.2 Preparation and Administration
Preparation
Measure the dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon, accurate to the fraction of the dose to be administered.
Once the measured dose has been removed from the XURIDEN packet, discard the unused portion of granules. Do not use any granules left in the open packet.
Administration with Food
- Place 3 to 4 ounces of applesauce, pudding or yogurt in a small clean container.
- Mix the measured amount of granules in the applesauce, pudding or yogurt
- Swallow applesauce/pudding/yogurt immediately. Do not chew the granules. Do not save the applesauce/pudding/yogurt for later use.
- Drink at least 4 ounces of water.
Administration in Milk or Infant Formula
XURIDEN can be mixed with milk or infant formula instead of the soft foods described above for patients receiving up to 3/4 teaspoon (2 grams) of XURIDEN. After weighing the dose of XURIDEN:
- Pour 5 mL of milk or infant formula into a 30 mL medicine cup.
- Insert the tip of the oral syringe into the medicine cup and draw up 5 mL of milk/infant formula into the syringe.
- Hold the syringe with the tip pointing upward. Pull down on the plunger until the plunger reaches 10 mL. This will add air to the syringe.
- Place the cap over the tip of the syringe. Then invert the syringe so the syringe tip is pointing down, and remove the plunger.
- Pour the measured amount of XURIDEN granules into the syringe barrel and reinsert the syringe plunger. Do not push up on the plunger.
- Gently swirl the syringe to mix the XURIDEN granules with the liquid.
- Turn the syringe so the syringe tip is pointing up. Then remove the syringe cap and push up on the plunger until the plunger reaches the 5 mL mark. This will remove air from the syringe.
- Place the tip of the syringe in the patient's mouth between the cheek and gum at the back of the mouth. Gently push the plunger all the way down.
- Refill the syringe with another 5 mL of milk/infant formula.
- Gently swirl the syringe to rinse any remaining XURIDEN granules from the syringe barrel.
- Place the tip of the syringe in the patient's mouth between the cheek and gum at the back of the mouth. Gently push the plunger all the way down.
- Follow with a bottle of milk or infant formula, if desired.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions and using a wide range of doses, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of XURIDEN was assessed in 4 patients with hereditary orotic aciduria ranging in age from 3 to 19 years (3 male, 1 female) who received 60 mg/kg of XURIDEN once daily for six weeks. All patients continued to receive XURIDEN for at least 24 months at dosages of up to 120 mg/kg once daily. No adverse reactions were reported with XURIDEN.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on XURIDEN use in pregnant women to inform a drug-associated risk. When administered orally to pregnant rats during the period of organogenesis, uridine triacetate at doses similar to the maximum recommended human dose (MRHD) of 120 mg/kg per day was not teratogenic and did not produce adverse effects on embryo-fetal development [see Data] .
The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study, uridine triacetate was administered orally to pregnant rats during the period of organogenesis at doses up to 2000 mg/kg per day (about 2.7 times the maximum recommended human dose (MRHD) of 120 mg/kg per day on a body surface area basis). There was no evidence of teratogenicity or harm to the fetus and no effect on maternal body weight and overall health.
8.2 Lactation
Risk Summary
There are no data on the presence of uridine triacetate in human milk, the effect on the breastfed infant or the effect on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for XURIDEN and any potential adverse effects on the breastfed infant from XURIDEN or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of XURIDEN have been established in pediatric patients. Use of XURIDEN is supported by a single open-label clinical trial of uridine triacetate in 4 patients and a retrospective review of the clinical course of 18 patients with hereditary orotic aciduria who were treated with uridine beginning at ages 2 months to 12 years. There are no apparent differences in clinical response between adults and pediatric patients with hereditary orotic aciduria treated with uridine, however, data are limited [see Clinical Studies (14)] .
-
11 DESCRIPTION
XURIDEN (uridine triacetate) oral granules is a pyrimidine analog indicated for uridine replacement therapy. Uridine triacetate has the chemical designation (2',3',5'-tri-O-acetyl-β-D-ribofuranosyl)-2,4(1H,3H)-pyrimidinedione. The molecular weight is 370.3 and it has an empirical formula of C 15H 18N 2O 9. The structural formula is:
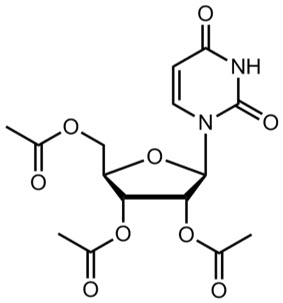
Each single-use 2-gram packet of XURIDEN orange-flavored oral granules (95% w/w) contains 2 grams of uridine triacetate and the following inactive ingredients: ethylcellulose (0.062 grams), Opadry Clear [proprietary dispersion of hydroxypropylmethylcellulose and Macrogol] (0.015 grams), and natural orange juice flavor (0.026 grams).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Uridine triacetate is an acetylated form of uridine. Following oral administration, uridine triacetate is deacetylated by nonspecific esterases present throughout the body, yielding uridine in the circulation (Figure 1).
Figure 1: Uridine Triacetate Conversion to Uridine
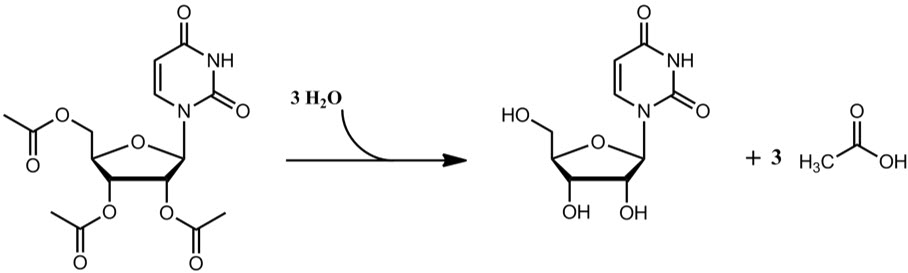
XURIDEN provides uridine in the systemic circulation of patients with hereditary orotic aciduria who cannot synthesize adequate quantities of uridine due to a genetic defect in uridine nucleotide synthesis.
12.2 Pharmacodynamics
Hereditary orotic aciduria (uridine monophosphate synthase deficiency) is a rare congenital autosomal recessive disorder of pyrimidine metabolism caused by a defect in uridine monophosphate synthase (UMPS). The UMPS gene encodes uridine 5′monophosphate synthase, a bifunctional enzyme that catalyzes the final two steps of the de novo pyrimidine biosynthetic pathway in mammalian cells.
The defect in UMP synthase in hereditary orotic aciduria has two primary biochemical consequences. First, the blockade of de novo UMP synthesis results in a systemic deficiency of pyrimidine nucleotides, accounting for most clinical consequences of the disease. Second, orotic acid from the de novo pyrimidine pathway that cannot be converted to UMP is excreted in the urine, accounting for the common name of the disorder, orotic aciduria. Orotic acid crystals in the urine can cause episodes of obstructive uropathy.
XURIDEN delivers uridine into the circulation, where it can be used by essentially all cells to make uridine nucleotides, compensating for the genetic deficiency in synthesis in patients with hereditary orotic aciduria. When intracellular uridine nucleotides are restored into the normal range, overproduction of orotic acid is reduced by feedback inhibition, so that urinary excretion of orotic acid is also reduced.
12.3 Pharmacokinetics
Absorption
XURIDEN delivers 4- to 6-fold more uridine into the systemic circulation compared to equimolar doses of uridine itself. Maximum concentrations of uridine in plasma following oral XURIDEN are generally achieved within 2 to 3 hours, and the half-life ranges from approximately 2 to 2.5 hours.
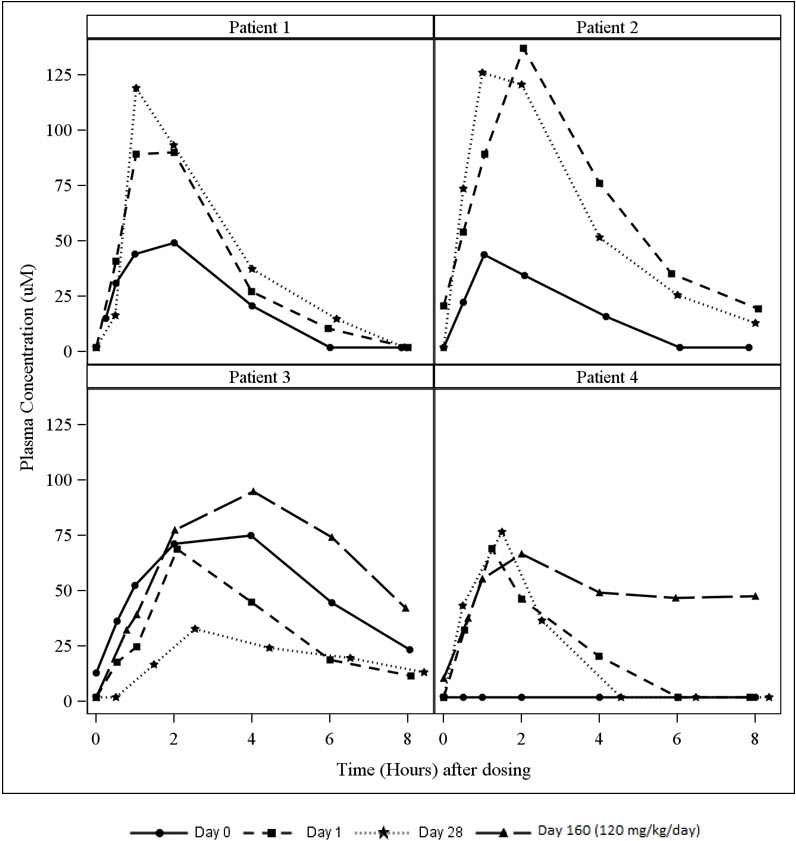
A study in patients with hereditary orotic aciduria included an assessment of plasma uridine pharmacokinetics in 4 patients. Three of the patients were previously treated with oral uridine. On Day 0 (baseline), these three patients received their usual daily dose of oral uridine as a single dose (150 to 200 mg/kg once daily) and on Day 1, initiated oral XURIDEN treatment (60 mg/kg once daily). A fourth patient was enrolled who was naïve to uridine replacement therapy. The dose of XURIDEN was increased on Day 116 to 120 mg/kg once daily in two patients (Patients 3 and 4) and plasma uridine concentrations were assessed on Day 160 (44 days after the dose increase).
Plasma uridine levels in all four patients are depicted in Figure 2. Pharmacokinetic parameters are summarized in Table 3. Mean exposure to plasma uridine as assessed by C maxand AUC was greater after oral XURIDEN than after oral uridine (approximately 4-fold on an equiweight basis, and 6-fold on an equimolar basis), although individual differences in relative bioavailability were noted. Plasma concentrations of the uridine catabolite uracil were generally below the limit of quantitation in all patients.
Table 3: Pharmacokinetic Parameters for Plasma Uridine Pharmacokinetic Parameters
(Plasma Uridine)Day 0 (Baseline) (Oral Uridine, 150 to 200 mg/kg once daily)
N = 3 *Day 1 (Oral XURIDEN, 60 mg/kg once daily)
N = 4Day 28 (Oral XURIDEN, 60 mg/kg once daily)
N = 4Day 160 (Oral XURIDEN, 120 mg/kg once daily)
N = 2 †- * Data shown are from patients previously treated with oral uridine
- † The dose of XURIDEN was increased on Day 116 to 120 mg/kg per day. Serial plasma samples were taken on Day 160 (44 days after the dose increase) for plasma uridine levels.
- ‡ T max range is expressed as the minimum and maximum values obtained
C max(µM)
mean ± SD56.0 ± 16.6 91.3 ± 32.2 88.7 ± 43.2 80.9 ± 20.0 T max(hours)
median (range ‡)2.0 (1.0, 4.0) 2.0 (1.2, 2.1) 1.3 (1.0, 2.5) 3.0 (2.0, 4.0) t ½(hours)
mean ± SD1.6 ± 0.7 1.6 ± 0.6 2.3 ± 1.6 8.2 ± 6.8 AUC (0-8)(µM∙hr)
mean ± SD238.0 ± 163.2 311.2 ± 153.3 278.7 ± 148.5 465.6 ± 95.3 Figure 2 Plasma Uridine Following Oral Administration of Uridine (Day 0) or XURIDEN (Days 1, 28 and 160) in Patients with Hereditary Orotic Aciduria
Distribution
Circulating uridine is taken up into mammalian cells via specific nucleoside transporters, and also crosses the blood brain barrier.
Excretion
Uridine can be excreted via the kidneys, but is also metabolized by normal pyrimidine catabolic pathways present in most tissues.
Drug Interaction Studies
In vitroenzyme inhibition data did not reveal meaningful inhibitory effects of uridine triacetate or uridine on CYP3A4, CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP2E1. In vitroenzyme induction data did not reveal an inducing effect of uridine triacetate or uridine on CYP1A2, CYP2B6, or CYP3A4.
In vitrodata showed that uridine triacetate was a weak substrate for P-glycoprotein. Uridine triacetate inhibited the transport of a known P-glycoprotein substrate, digoxin, with an IC50 of 344 µM. Due to the potential for high local (gut) concentrations of the drug after dosing, the interaction of XURIDEN with orally administered P-gp substrate drugs cannot be ruled out.
In vivodata in humans are not available.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of uridine triacetate.
Uridine triacetate was not genotoxic in the Ames test, the mouse lymphoma assay and the mouse micronucleus test.
Orally administered uridine triacetate did not affect fertility or general reproductive performance in male and female rats at doses up to 2000 mg/kg per day (about 2.7 times the maximum recommended human dose (MRHD) of 120 mg/kg per day on a body surface area basis).
-
14 CLINICAL STUDIES
The efficacy of XURIDEN was evaluated in a 6-week open-label study in 4 patients with hereditary orotic aciduria (3 male, 1 female; age range from 3 to 19 years). Three patients were previously treated with uridine and were switched at study entry to XURIDEN. All patients were administered XURIDEN orally at a daily dosage of 60 mg/kg once daily. Following the initial 6-weeks, all 4 patients continued to receive XURIDEN daily in the extension phase of the study at dosages of 60 to 120 mg/kg for a total duration of 24 months.
The study assessed changes in the patients' pre-specified hematologic parameters during the initial 6-week period and the extension phase. The pre-specified hematologic parameters were: neutrophil count and percent neutrophils (Patient 1), white blood cell count (Patient 2), and mean corpuscular volume (Patients 3 and 4). For patients switched from oral uridine to oral XURIDEN (Patients 1, 2, and 3), the primary endpoint was stability of the hematologic parameter; for the treatment-naïve patient (Patient 4), the primary endpoint was improvement of the hematologic parameter.
Secondary endpoints were urine orotic acid and orotidine levels, and growth (height and weight) for all patients.
Table 4 summarizes the changes in the patients' pre-specified hematologic parameters at Week 6 and 24 Months compared to baseline. After 6 weeks of treatment, Patients 1 and 3 met the pre-specified criteria for stability of the hematologic parameter. When Patient 2 was switched from uridine to XURIDEN treatment, the pre-specified parameter of white blood cell count remained stable; however, documentation of a low white blood cell count prior to uridine initiation was not available. Patient 4 did not meet the pre-specified endpoint of improvement of the hematologic parameter.
By 24 months of treatment with XURIDEN, the pre-specified hematologic parameter for Patient 1 worsened, but the assessment was confounded by Cohen syndrome with associated neutropenia, which was diagnosed after the patient completed the study. The pre-specified hematologic parameters for Patients 2, 3, and 4 remained essentially unchanged.
Table 4: Pre-Specified Hematologic Parameters at Baseline, 6 Weeks and 24 Months in XURIDEN-Treated Patients with Hereditary Orotic Aciduria Patient Pre-specified Hematologic Parameter
(Age-specific reference range)Baseline
(Day 0)6 Weeks
(% Change from Baseline)24 Months
(% Change from Baseline)Patient 1 Neutrophil Count
(1.5 to 8.0 x10 3/mm 3)0.95 0.81
(-15%)0.61
(-36%)Neutrophil %
(26 to 48%)21 23
(10%)13
(-38%)Patient 2 White Blood Cell Count
(3.8 to 10.6 x10 9/L)7.8 7.4
(-5%)8.6
(10%)Patient 3 Mean Corpuscular Volume
(75 to 91 fL)109.9 108.5
(-1%)108.8
(-1%)Patient 4 Mean Corpuscular Volume
(72 to 90 fL)114.6 113.4
(-1%)112.9
(-1.5%)At baseline, Patients 1, 2, and 3, previously treated with uridine, had normal urine orotic acid levels, and those remained stable after 6 weeks of treatment with XURIDEN. All four patients had normal urine orotidine levels at baseline which remained stable after 6 weeks of treatment with XURIDEN. After 24 months of treatment, urine orotic acid and orotidine levels remained stable in all four patients.
The treatment effect of XURIDEN on growth was assessed in the three pediatric patients (Patients 1, 3, and 4). At baseline, weight and height were at or below the lower limit of normal for age for all three patients. After 24 months of treatment, each patient's growth parameters remained essentially unchanged.
Case reports
Nineteen (19) case reports of patients with hereditary orotic aciduria have been documented in published literature. Eighteen (18) patients were diagnosed as infants or children between the ages of 2 months and 12 years and were treated with exogenous sources of uridine. One patient, diagnosed at age 28, was not treated with exogenous uridine.
All 19 patients presented with significantly elevated levels of urinary orotic acid. Fifteen of 19 had abnormal hematologic parameters at presentation, including 15 with megaloblastic anemia, 8 with leukopenia and at least 2 with neutropenia. Oral administration of exogenous sources of uridine was reported to significantly improve hematologic abnormalities (megaloblastic anemia, leukopenia and neutropenia) within 2 to 3 weeks in almost all documented cases when administered in sufficient amounts. Concentrations of urinary orotic acid were significantly reduced within 1 to 2 weeks of initiating uridine replacement therapy. Some fluctuation in levels of urinary orotic acid were observed, but always at much lower levels than those reported prior to treatment. Improvements in body weight were also documented over time with continued uridine replacement therapy.
The effects of exogenous uridine were maintained over months and years, as long as treatment continued at sufficient doses (with appropriate dose increases based on body weight increases). Most hematologic abnormalities and orotic aciduria reappeared within days up to 2 or 3 weeks when administration of uridine was stopped or the dose was reduced. If treatment was interrupted for longer periods, body weight growth receded. If absolute dosages were not adjusted adequately to compensate for body weight gains, signs and symptoms of hereditary orotic aciduria recurred.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
XURIDEN orange-flavored oral granules (95% w/w) are available in single-use packets (NDC: 50633-330-02) containing 2 grams of uridine triacetate in cartons of 30 packets each (NDC: 50633-330-30).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Instructions for Use)
Administration
Advise the patient or caregiver:
- To measure the prescribed dose using either a scale accurate to at least 0.1 gram, or a graduated teaspoon, accurate to the fraction of the dose to be administered.
- To discard the unused portion of granules in a packet after measuring out the dose.
- That XURIDEN can be taken mixed in food (applesauce, pudding or yogurt) or mixed in milk or infant formula.
- That the XURIDEN granules should not be chewed.
- SPL UNCLASSIFIED SECTION
-
Instructions for Use
XURIDEN
®(ZUR-uh-den)
(uridine triacetate)
oral granules
Read this Instructions for Use before you prepare XURIDEN for the first time, each time you get a refill, and as needed. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment. Ask your healthcare provider if you have any questions about how to mix or give a dose of XURIDEN the right way.
Important Information
- Take XURIDEN exactly as your healthcare provider tells you to.
- Your healthcare provider will prescribe the dose of XURIDEN depending on your body weight. Your healthcare provider will tell you the right dose to take.
- Your healthcare provider will show you how to measure the prescribed dose. The prescribed dose of XURIDEN can be measured using a scale or an adjustable measuring spoon.
- Your healthcare provider may change your dose if needed. Do not change the dose without first talking with your healthcare provider.
- XURIDEN may be taken by mixing XURIDEN with 3 to 4 ounces of applesauce, pudding or yogurt or may be mixed with milk or infant formula.
- XURIDEN mixed with applesauce, pudding or yogurt should be eaten right away. Do not save the mixed applesauce, pudding or yogurt for later use.
- Do notchew the XURIDEN oral granules.
For each dose of XURIDEN given in applesauce, pudding or yogurt, you will need the following (See Figure A):
- paper towels
- XURIDEN packet or packets containing the medicine needed for the prescribed dose
- 1 scale or 1 adjustable measuring spoon
- small spoon for stirring
- 1 small clean container (such as a small cup or bowl)
- 3 to 4 ounces of applesauce, pudding or yogurt
- 4 ounces of drinking water
Figure A 
Step 1: Choose a clean flat work surface. Place a clean paper towel on the work surface. Then place the other supplies on the paper towel. Step 2: Wash and dry your hands. Step 3: Place 3 to 4 ounces of soft food such as applesauce, pudding, or yogurt in the small clean cup or bowl. 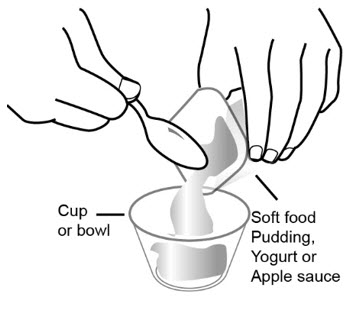
Step 4: Select the number of XURIDEN packets needed for the prescribed dose. 
Step 5: Measure the dose: -
If you are using a scale:
- Open the packet or packets and measure out the amount needed for the prescribed dose on the scale. Follow the instructions that came with the scale for correct and accurate use.
-
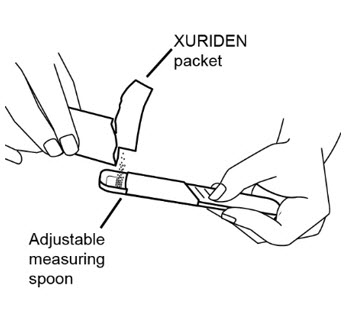
If you are using an adjustable measuring spoon:
- Open the packet or packets and use the adjustable measuring spoon to measure out the amount needed for the prescribed dose as shown.
- Throw away any unused XURIDEN in the trash. Do not use any XURIDEN left in the open packet.
Step 6: Sprinkle the XURIDEN onto the soft food. 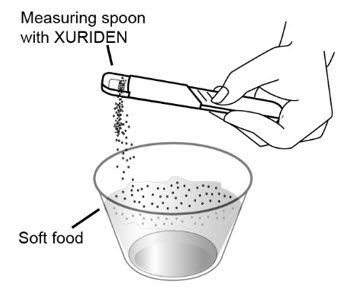
Step 7: Use the small spoon to mix the medicine and the applesauce, pudding, or yogurt together. 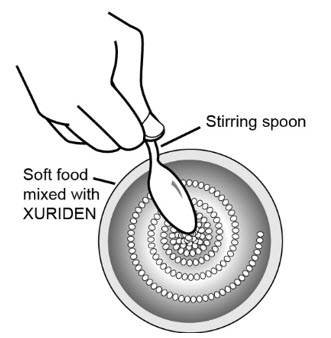
Step 8: Use the small spoon to give or take the soft food and XURIDEN mixture.
XURIDEN mixed with soft food should be eaten right away without chewing.To avoid a bitter taste from the medicine, do not chew the granules. Make sure all of the mixture is swallowed.
Do notsave the food for later use.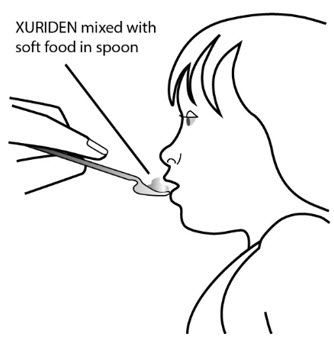
Step 9: Drink at least 4 ounces of water. 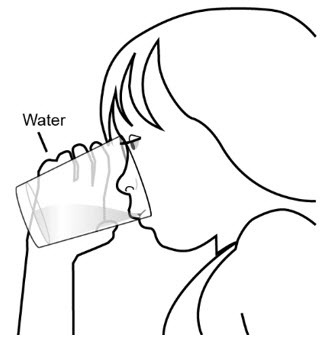
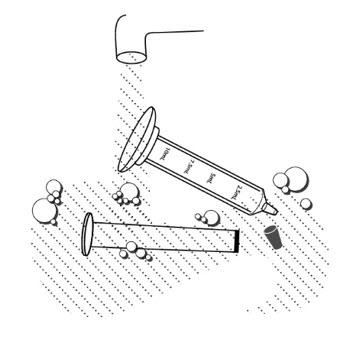
Step 10: Wash the supplies needed to give the dose as your healthcare provider has told you. Throw away the paper towel and clean the work surface. Wash your hands. For each dose of XURIDEN given in milk or formula to children receiving up to ¾ teaspoon (2 grams of XURIDEN), you will need the following (See Figure B):
- paper towels
- XURIDEN packet or packets containing the medicine needed for your prescribed dose
- 1 oral dosing syringe (10mL) with a cap
- 1 scale or 1 adjustable measuring spoon
- milk or infant formula (10mL)
- 1 medicine cup (30mL)
Figure B 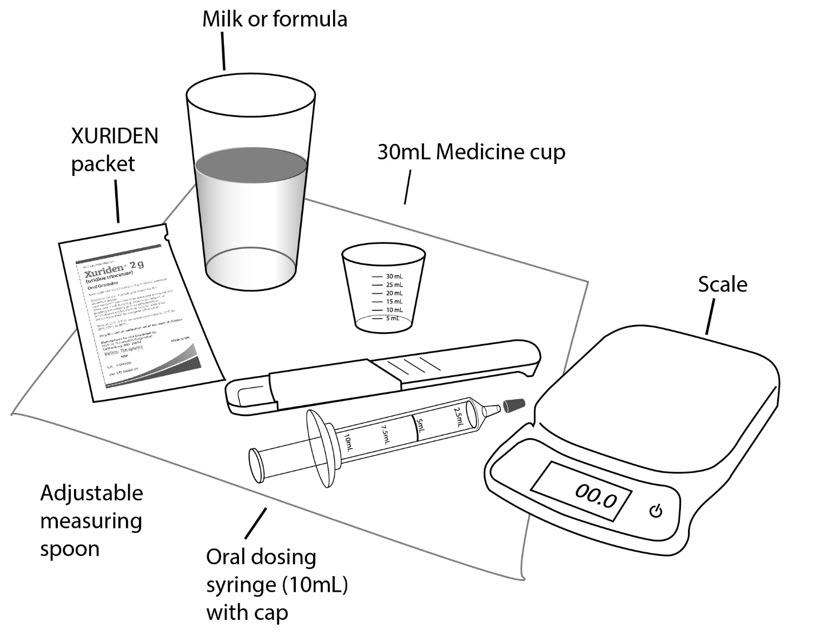
Step 1: Choose a clean flat work surface. Place a clean paper towel on the work surface. Then place the other supplies on the paper towel. - If using infant formula, prepare the formula according to the directions on the infant formula package.
Step 2: Wash and dry your hands. Step 3: Open one XURIDEN packet. 
Step 4: Measure the dose: -
If you are using a scale:
- Open the packet and measure out the amount needed for the prescribed dose on the scale. Follow the instructions that came with the scale for correct and accurate use.
-
If you are using an adjustable measuring spoon:
- Open the packet and use the adjustable measuring spoon to measure out the amount needed for the prescribed dose as shown.
- Throw away any unused XURIDEN in the trash. Do not use any XURIDEN left in the open packet.
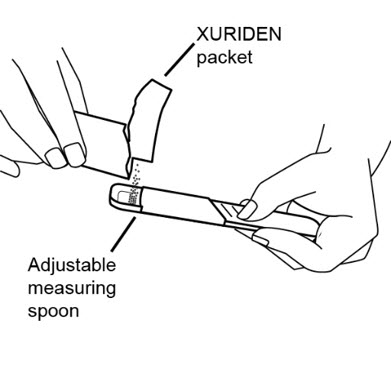
Step 5: Pour 5 mL of either milk or infant formula into the 30mL medicine cup. 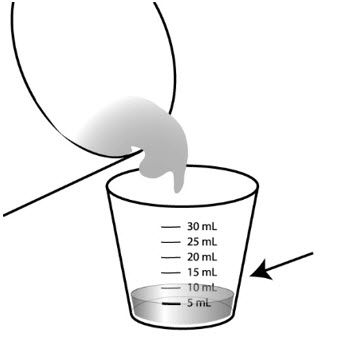
Step 6: Place the syringe tip into the medicine cup. Pull up on the plunger until all the liquid is removed from the cup and the plunger reaches the 5 mL line on the syringe. 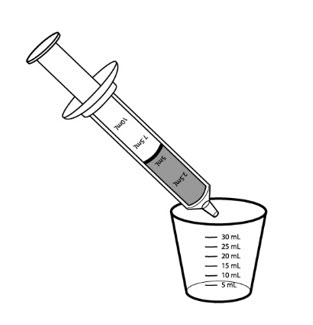
Step 7: Hold the syringe with the tip pointing upward. Pull down on the plunger until the plunger reaches the 10 mL line on the syringe. This will add air to the syringe. 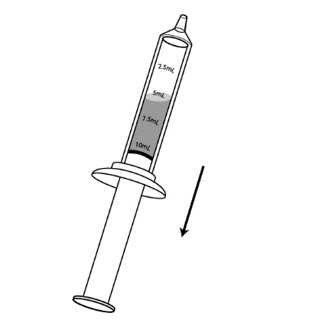
Step 8: Place the syringe cap over the tip of the syringe. Now hold the syringe so the tip is pointing down and remove the plunger. 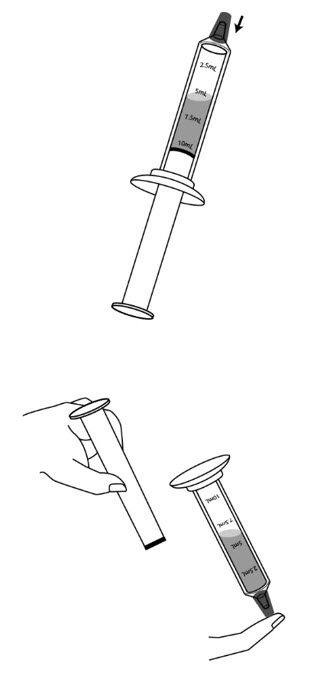
Step 9: Pour the measured amount of XURIDEN granules into the syringe barrel, and put the plunger back in. Do not push further on the plunger. 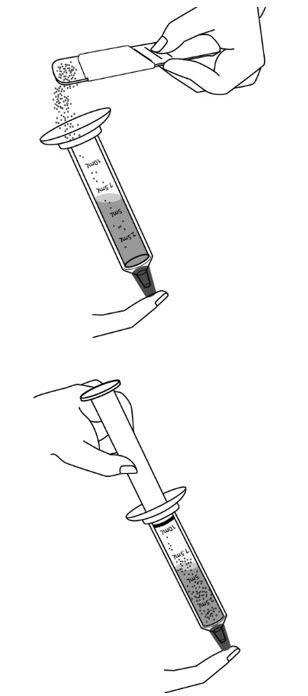
Step 10: Gently swirl the syringe to mix the XURIDEN with the liquid. 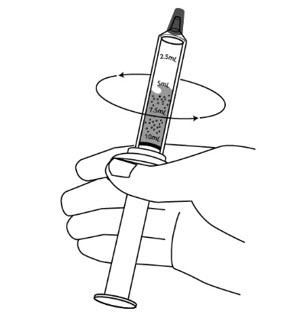
Step 11: Turn the syringe so the syringe tip is pointing up, then remove the syringe cap, and push up on the plunger until the plunger reaches the 5mL line on the syringe. This will remove the air from the syringe. 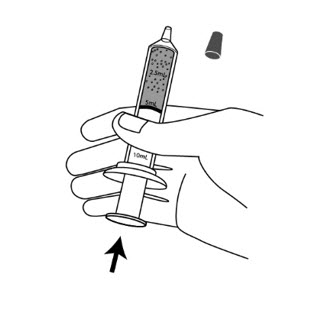
Step 12: Give the mixture to the child right awayto avoid a bitter taste from the medicine. Place the tip of the syringe in your child's mouth between the cheek and the gum at the back of the mouth. Gently push the plunger all the way down. 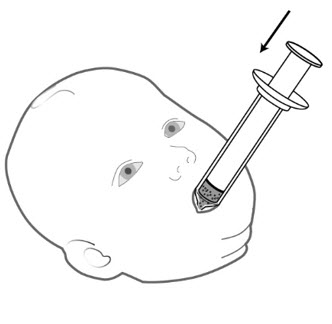
Step 13: Pour another 5 mL of milk or formula into the medicine cup. Step 14: Refill the syringe by placing the syringe tip into the medicine cup. Pull up on the plunger until all the liquid is removed from the cup and the plunger reaches the 5 mL line on the syringe. Step 15: Gently swirl the syringe to make sure any medicine remaining in the syringe is mixed with the liquid. Step 16: Give the liquid to the child right away. Place the tip of the syringe in your child's mouth between the cheek and the gum at the back of the mouth. Gently push the plunger all the way down. Step 17: Give your child a bottle of milk or formula after giving the XURIDEN dose if you wish to. Step 18: Remove the plunger from the barrel of the dosing syringe and wash the syringe and mixing cup, with warm water and dish soap. Rinse with water and air dry. - Wash the supplies needed to measure your dose as your healthcare provider has told you.
- Throw away the paper towel and clean the work surface.
- Wash your hands.
How should I store XURIDEN?
- Store XURIDEN at room temperature between 59° F to 86°F (15° to 30°C).
- Keep XURIDEN and all medicines out of the reach of children.
General information about the safe and effective use of XURIDEN
This Instructions for Use leaflet summarizes the most important information about XURIDEN. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about XURIDEN that is written for healthcare professionals.
For more information, go to www.XURIDEN.com.
What are the ingredients in XURIDEN?
Active ingredient:uridine triacetate
Inactive ingredients:ethylcellulose, hydroxypropylmethylcellulose, Macrogol, natural orange juice flavor
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for and distributed by
BTG International Inc,
West Conshohocken, PA, 19428
XURIDEN ®is a registered trademark of BTG International Inc.
Issued: 08/2023
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2 g Packet Label
NDC: 50633-330-02
RxOnlyXuriden ®2 g
(uridine triacetate)Oral Granules
Packet contains 1 x 2 gram packets
BTG International Inc

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 Count Carton Label
NDC: 50633-330-30
RxOnlyXuriden ®2 g
(uridine triacetate)Oral Granules
Carton contains 30 x 2 gram packets
BTG International Inc
-
INGREDIENTS AND APPEARANCE
XURIDEN
uridine triacetate granuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50633-330 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength URIDINE TRIACETATE (UNII: 2WP61F175M) (URIDINE - UNII:WHI7HQ7H85) URIDINE TRIACETATE 951 mg in 1 g Inactive Ingredients Ingredient Name Strength ORANGE JUICE (UNII: 5A9KE2L9L3) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) Product Characteristics Color white (white to off-white) Score Shape Size Flavor ORANGE (Natural Orange Juice Flavor S.D. #80618) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50633-330-30 30 in 1 CARTON 05/31/2024 1 NDC: 50633-330-02 2 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208169 05/31/2024 Labeler - BTG International Inc (617382395) Establishment Name Address ID/FEI Business Operations Butterworth Laboratories Limited 225081538 analysis(50633-330) Establishment Name Address ID/FEI Business Operations Almac Sciences 232665666 api manufacture(50633-330) , analysis(50633-330) Establishment Name Address ID/FEI Business Operations Almac Pharma Services 233170864 manufacture(50633-330) , analysis(50633-330) , pack(50633-330) , label(50633-330)
Trademark Results [XURIDEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XURIDEN 85386985 4822320 Live/Registered |
Wellstat Therapeutics Corporation 2011-08-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.