NEXLETOL- bempedoic acid tablet, film coated
Nexletol by
Drug Labeling and Warnings
Nexletol by is a Prescription medication manufactured, distributed, or labeled by Esperion Therapeutics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NEXLETOL™ safely and effectively. See full prescribing information for NEXLETOL.
NEXLETOL (bempedoic acid) tablets, for oral use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
NEXLETOL is an adenosine triphosphate-citrate lyase (ACL) inhibitor indicated as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who require additional lowering of LDL-C. (1)
Limitations of Use: The effect of NEXLETOL on cardiovascular morbidity and mortality has not been determined. (1)
DOSAGE AND ADMINISTRATION
Administer 180 mg orally once daily with or without food. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 180 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Hyperuricemia: Elevations in serum uric acid have occurred. Assess uric acid levels periodically as clinically indicated. Monitor for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate. (5.1)
- Tendon Rupture: Tendon rupture has occurred. Discontinue NEXLETOL at the first sign of tendon rupture. Avoid NEXLETOL in patients who have a history of tendon disorders or tendon rupture. (5.2)
ADVERSE REACTIONS
Most common (incidence ≥ 2% and greater than placebo) adverse reactions are upper respiratory tract infection, muscle spasms, hyperuricemia, back pain, abdominal pain or discomfort, bronchitis, pain in extremity, anemia, and elevated liver enzymes. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Esperion at 833-377-7633 (833 ESPRMED) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyperuricemia
5.2 Tendon Rupture
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyperuricemia
NEXLETOL inhibits renal tubular OAT2 and may increase blood uric acid levels [see Clinical Pharmacology (12.3)]. In clinical trials, 26% of NEXLETOL-treated patients with normal baseline uric acid values (versus 9.5% placebo) experienced hyperuricemia one or more times, and 3.5% of patients experienced clinically significant hyperuricemia reported as an adverse reaction (versus 1.1% placebo). Increases in uric acid levels usually occurred within the first 4 weeks of treatment initiation and persisted throughout treatment. After 12 weeks of treatment, the mean placebo-adjusted increase in uric acid compared to baseline was 0.8 mg/dL for patients treated with NEXLETOL.
Elevated blood uric acid may lead to the development of gout. Gout was reported in 1.5% of patients treated with NEXLETOL and 0.4% of patients treated with placebo. The risk for gout events was higher in patients with a prior history of gout (11.2% NEXLETOL versus 1.7% placebo), although gout also occurred more frequently than placebo in patients treated with NEXLETOL who had no prior gout history (1.0% NEXLETOL versus 0.3% placebo).
Advise patients to contact their healthcare provider if symptoms of hyperuricemia occur. Assess serum uric acid when clinically indicated. Monitor patients for signs and symptoms of hyperuricemia, and initiate treatment with urate-lowering drugs as appropriate.
5.2 Tendon Rupture
NEXLETOL is associated with an increased risk of tendon rupture or injury. In clinical trials, tendon rupture occurred in 0.5% of patients treated with NEXLETOL versus 0% of placebo-treated patients and involved the rotator cuff (the shoulder), biceps tendon, or Achilles tendon. Tendon rupture occurred within weeks to months of starting NEXLETOL. Tendon rupture may occur more frequently in patients over 60 years of age, in those taking corticosteroid or fluoroquinolone drugs, in patients with renal failure, and in patients with previous tendon disorders.
Discontinue NEXLETOL immediately if the patient experiences rupture of a tendon. Consider discontinuing NEXLETOL if the patient experiences joint pain, swelling, or inflammation. Advise patients to rest at the first sign of tendinitis or tendon rupture and to contact their healthcare provider if tendinitis or tendon rupture symptoms occur. Consider alternative therapy in patients with a history of tendon disorders or tendon rupture.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hyperuricemia [see Warnings and Precautions (5.1)]
- Tendon Rupture [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to NEXLETOL in two placebo-controlled trials that included 2009 patients treated with NEXLETOL for 52 weeks (median treatment duration of 52 weeks) [see Clinical Studies (14)]. The mean age for NEXLETOL-treated patients was 65.4 years, 29% were women, 3% were Hispanic, 95% White, 3% Black, 1% Asian, and 1% other races. All patients received NEXLETOL 180 mg orally once daily plus maximally tolerated statin therapy alone or in combination with other lipid-lowering therapies. At baseline, 97% of patients had clinical atherosclerotic cardiovascular disease (ASCVD) and about 4% had a diagnosis of heterozygous familial hypercholesterolemia (HeFH). Patients on simvastatin 40 mg/day or higher were excluded from the trials.
Adverse reactions led to discontinuation of treatment in 11% of NEXLETOL-treated patients and 8% of placebo-treated patients. The most common reasons for NEXLETOL treatment discontinuation were muscle spasms (0.5% versus 0.3% placebo), diarrhea (0.4% versus 0.1% placebo), and pain in extremity (0.3% versus 0.0% placebo). Adverse reactions reported in at least 2% of NEXLETOL-treated patients and more frequently than in placebo-treated patients are shown in Table 1.
Table 1. Adverse Reactions (≥ 2% and Greater than placebo) in NEXLETOL-Treated Patients with ASCVD and HeFH (Studies 1 and 2) Adverse Reaction NEXLETOL + Statin and ± Other Lipid Lowering Therapies
(N = 2009)
%Placebo
(N = 999)
%- * Hyperuricemia includes hyperuricemia and blood uric acid increased.
- † Abdominal pain or discomfort includes abdominal pain, abdominal pain upper, abdominal pain lower, and abdominal discomfort.
- ‡ Elevated liver enzymes includes AST increased, ALT increased, hepatic enzyme increased, and liver function test increased.
Upper respiratory tract infection 4.5 4.0 Muscle spasms 3.6 2.3 Hyperuricemia* 3.5 1.1 Back pain 3.3 2.2 Abdominal pain or discomfort† 3.1 2.2 Bronchitis 3.0 2.5 Pain in extremity 3.0 1.7 Anemia 2.8 1.9 Elevated liver enzymes‡ 2.1 0.8 Tendon Rupture
NEXLETOL was associated with an increased risk of tendon rupture, occurring in 0.5% of NEXLETOL-treated patients versus 0% of placebo-treated patients.
Gout
NEXLETOL was associated with an increased risk of gout, occurring in 1.5% of NEXLETOL-treated patients versus 0.4% of placebo-treated patients.
Benign Prostatic Hyperplasia
NEXLETOL was associated with an increased risk of benign prostatic hyperplasia (BPH) or prostatomegaly in men with no reported history of BPH, occurring in 1.3% of NEXLETOL-treated patients versus 0.1% of placebo-treated patients. The clinical significance is unknown.
Atrial Fibrillation
NEXLETOL was associated with an imbalance in atrial fibrillation, occurring in 1.7% of NEXLETOL-treated patients versus 1.1% of placebo-treated patients.
Laboratory Tests
NEXLETOL was associated with persistent changes in multiple laboratory tests within the first 4 weeks of treatment. Laboratory test values returned to baseline following discontinuation of treatment.
Increase in Creatinine and Blood Urea Nitrogen: Overall, there was a mean increase in serum creatinine of 0.05 mg/dL compared to baseline with NEXLETOL at Week 12. Approximately 3.8% of patients treated with NEXLETOL had blood urea nitrogen values that doubled (versus 1.5% placebo), and about 2.2% of patients had creatinine values that increased by 0.5 mg/dL (versus 1.1% placebo).
Decrease in Hemoglobin and Leukocytes: Approximately 5.1% of patients (versus 2.3% placebo) had decreases in hemoglobin levels of 2 or more g/dL and below the lower limit of normal on one or more occasion. Anemia was reported in 2.8% of patients treated with NEXLETOL and 1.9% of patients treated with placebo. Hemoglobin decrease was generally asymptomatic and did not require medical intervention. Decreased leukocyte count was also observed. Approximately 9.0% of NEXLETOL-treated patients with normal baseline leukocyte count had a decrease to less than the lower limit of normal on one or more occasion (versus 6.7% placebo). Leukocyte decrease was generally asymptomatic and did not require medical intervention. In clinical trials, there was a small imbalance in skin or soft tissue infections, including cellulitis (0.8% versus 0.4%), but there was no imbalance in other infections.
Increase in Platelet Count: Approximately 10.1% of patients (versus 4.7% placebo) had increases in platelet counts of 100× 109/L or more on one or more occasion. Platelet count increase was asymptomatic, did not result in increased risk for thromboembolic events, and did not require medical intervention.
Increase in Liver Enzymes: Increases in hepatic transaminases (AST and/or ALT) were observed with NEXLETOL. In most cases, the elevations were transient and resolved or improved with continued therapy or after discontinuation of therapy. Increases to more than 3× the upper limit of normal (ULN) in AST occurred in 1.4% of patients treated with NEXLETOL versus 0.4% of placebo patients, and increases to more than 5× ULN occurred in 0.4% of NEXLETOL-treated versus 0.2% of placebo-treated patients. Increases in ALT occurred with similar incidence between NEXLETOL- and placebo-treated patients. Elevations in transaminases were generally asymptomatic and not associated with elevations ≥2× ULN in bilirubin or with cholestasis.
-
7 DRUG INTERACTIONS
Simvastatin Clinical Impact: Concomitant use of NEXLETOL with simvastatin causes an increase in simvastatin concentration and may increase the risk of simvastatin-related myopathy [see Clinical Pharmacology (12.3)]. Intervention: Avoid concomitant use of NEXLETOL with simvastatin greater than 20 mg. Pravastatin Clinical Impact: Concomitant use of NEXLETOL with pravastatin causes an increase in pravastatin concentration and may increase the risk of pravastatin-related myopathy [see Clinical Pharmacology (12.3)]. Intervention: Avoid concomitant use of NEXLETOL with pravastatin greater than 40 mg. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Discontinue NEXLETOL when pregnancy is recognized unless the benefits of therapy outweigh the potential risks to the fetus.
There are no available data on NEXLETOL use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, bempedoic acid was not teratogenic in rats and rabbits when administered at doses resulting in exposures up to 11 and 12 times, respectively, the human exposures at the maximum clinical dose, based on AUC (see Data). NEXLETOL decreases cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol; therefore, NEXLETOL may cause fetal harm when administered to pregnant women based on the mechanism of action [see Clinical Pharmacology (12.1)]. In addition, treatment of hyperlipidemia is not generally necessary during pregnancy. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hyperlipidemia for most patients.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Bempedoic acid was not teratogenic when given orally at doses of 60 and 80 mg/kg/day, resulting in 11 and 12 times the systemic exposure in humans at the maximum recommended human dose (MRHD) of 180 mg to pregnant rats and rabbits, respectively. In an embryofetal development study in rats, bempedoic acid was given orally to pregnant rats at 10, 30, and 60 mg/kg/day during the period of organogenesis from gestation day 6 to 17. There were increases in the incidence of non-adverse fetal skeletal variations (bent long bones and bent scapula and incomplete ossification) at doses ≥ 10 mg/kg/day (less than the clinical exposure) in the absence of maternal toxicity. At maternally toxic doses, bempedoic acid caused decreases in the numbers of viable fetuses, increases in post-implantation loss, and increased total resorptions at 60 mg/kg/day (11 times MRHD) and reduced fetal body weight at ≥ 30 mg/kg/day (4 times the MRHD). No adverse development effects were observed when bempedoic acid was given to pregnant rabbits during the period of organogenesis (gestation day 6 to 18) at doses up to 80 mg/kg/day (12 times MRHD).
In a pre- and post-natal development study in pregnant rats given oral doses of bempedoic acid at 5, 10, 20, 30 and 60 mg/kg/day throughout pregnancy and lactation (gestation day 6 to lactation day 20), there were adverse effects on delivery in the presence of maternal toxicity, including: increases in stillborn pups, reductions in numbers of live pups, pup survival, pup growth and slight delays in learning and memory at ≥ 10 mg/kg/day (at exposures equivalent to the MRHD).
8.2 Lactation
Risk Summary
There is no information regarding the presence of NEXLETOL in human or animal milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. NEXLETOL decreases cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol and may cause harm to the breastfed infant. Because of the potential for serious adverse reactions in a breastfed infant, based on the mechanism of action, advise patients that breastfeeding is not recommended during treatment with NEXLETOL [see Use in Specific Populations (8.1), Clinical Pharmacology (12.1)].
8.4 Pediatric Use
The safety and effectiveness of NEXLETOL have not been established in pediatric patients.
8.5 Geriatric Use
Of the 3009 patients in clinical trials of NEXLETOL, 1753 (58%) were 65 years and older, while 478 (16%) were 75 years and older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dosage adjustment is necessary in patients with mild or moderate renal impairment. There is limited experience with NEXLETOL in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2), and NEXLETOL has not been studied in patients with end-stage renal disease (ESRD) receiving dialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment is necessary in patients with mild or moderate hepatic impairment (Child-Pugh A or B) [see Clinical Pharmacology (12.3)]. Patients with severe hepatic impairment (Child-Pugh C) have not been studied.
- 10 OVERDOSAGE
-
11 DESCRIPTION
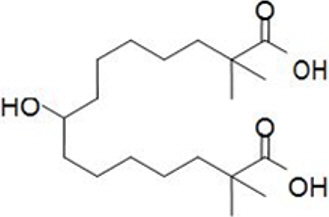
NEXLETOL tablets, for oral use, contain bempedoic acid, an adenosine triphosphate-citrate lyase (ACL) inhibitor. The chemical name for bempedoic acid is 8-hydroxy-2,2,14,14-tetramethyl-pentadecanedioic acid. The molecular formula is C19H36O5, and the molecular weight is 344.5 grams per mole. Bempedoic acid is a white to off-white crystalline powder that is highly soluble in ethanol, isopropanol and pH 8 phosphate buffer, and insoluble in water and aqueous solutions below pH 5.
Structural formula:
Each film-coated tablet of NEXLETOL contains 180 mg of bempedoic acid and the following inactive ingredients: colloidal silicon dioxide, hydroxyl propyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The film coating comprises of partially hydrolyzed polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bempedoic acid is an adenosine triphosphate-citrate lyase (ACL) inhibitor that lowers low-density lipoprotein cholesterol (LDL-C) by inhibition of cholesterol synthesis in the liver. ACL is an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway. Bempedoic acid and its active metabolite, ESP15228, require coenzyme A (CoA) activation by very long-chain acyl-CoA synthetase 1 (ACSVL1) to ETC-1002-CoA and ESP15228-CoA, respectively. ACSVL1 is expressed primarily in the liver. Inhibition of ACL by ETC-1002-CoA results in decreased cholesterol synthesis in the liver and lowers LDL-C in blood via upregulation of low-density lipoprotein receptors.
12.2 Pharmacodynamics
Administration of bempedoic acid in combination with maximally tolerated statins, with or without other lipid modifying agents, decreases LDL-C, non-high density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (apo B), and total cholesterol (TC) in patients with hyperlipidemia.
12.3 Pharmacokinetics
Bempedoic acid pharmacokinetic parameters are presented as the mean [standard deviation ± (SD)] unless otherwise specified. The steady-state maximum plasma concentration (Cmax) and area under the curve (AUC) following multiple-dose administration of bempedoic acid at 180 mg/day were 20.6 ± 6.1 µg/mL and 289.0 ± 96.4 µg∙h/mL, respectively. Bempedoic acid steady-state pharmacokinetics were generally linear over a range of > 60 mg to 220 mg (approximately 33% to 122% of the recommended dosage of 180 mg daily). There were no time-dependent changes in bempedoic acid pharmacokinetics following repeat administration at the recommended dosage, and bempedoic acid steady-state was achieved after 7 days. The mean accumulation ratio was approximately 2.3-fold.
The steady-state Cmax and AUC of the active metabolite (ESP15228) of bempedoic acid were 2.8 ± 0.9 µg/mL and 51.2 ± 17.2 µg∙h/mL, respectively. ESP15228 likely made a minor contribution to the overall clinical activity of bempedoic acid based on systemic exposure, relative potency, and pharmacokinetic properties.
Absorption
Pharmacokinetic data indicate that bempedoic acid is absorbed with a median time to maximum concentration of 3.5 hours when administered as NEXLETOL 180 mg tablets.
Distribution
The bempedoic acid apparent volume of distribution (V/F) was 18 L. Plasma protein binding of bempedoic acid, its glucuronide and its active metabolite, ESP15228, were 99.3%, 98.8% and 99.2%, respectively. Bempedoic acid does not partition into blood cells.
Elimination
The steady-state clearance (CL/F) of bempedoic acid was 11.2 mL/min after once-daily dosing; renal clearance of unchanged bempedoic acid represented less than 2% of total clearance. The mean ± SD half-life for bempedoic acid in humans was 21 ± 11 hours at steady-state.
Metabolism
The primary route of elimination for bempedoic acid is through metabolism of the acyl glucuronide. Bempedoic acid is also reversibly converted to an active metabolite (ESP15228) based on aldo-keto reductase activity observed in vitro from human liver. Mean plasma AUC metabolite/parent drug ratio for ESP15228 following repeat-dose administration was 18% and remained constant over time. Both compounds are converted to inactive glucuronide conjugates in vitro by UGT2B7. Bempedoic acid, ESP15228 and their respective conjugated forms were detected in plasma with bempedoic acid accounting for the majority (46%) of the AUC0-48h and its glucuronide being the next most prevalent (30%). ESP15228 and its glucuronide represented 10% and 11% of the plasma AUC0-48h, respectively.
Excretion
Following single oral administration of 240 mg of bempedoic acid (1.3 times the approved recommended dose), approximately 70% of the total dose (bempedoic acid and its metabolites) was recovered in urine, primarily as the acyl glucuronide conjugate of bempedoic acid, and approximately 30% was recovered in feces. Less than 5% of the administered dose was excreted as unchanged bempedoic acid in feces and urine combined.
Specific Populations
Patients with Renal Impairment
Pharmacokinetics of bempedoic acid was evaluated in a single-dose pharmacokinetic study in subjects with varying degrees of renal function. The mean bempedoic acid AUC in subjects with mild renal impairment (n = 8) were 1.5-fold higher compared to those with normal renal function (n = 6). Relative to those with normal renal function, mean bempedoic acid AUCs were higher in patients with moderate (n = 5) or severe (n = 5) renal impairment by 2.3-fold and 2.4-fold, respectively.
A population pharmacokinetic analysis was performed on pooled data from all clinical trials (n = 2261) to further evaluate the effects of renal function on the steady-state AUC of bempedoic acid. Compared to patients with normal renal function, the mean bempedoic acid exposures were higher in patients with mild or moderate renal impairment by 1.4-fold (90% CI: 1.3, 1.4) and 1.9-fold (90% CI: 1.7, 2.0), respectively. These differences were not clinically significant. Clinical studies of NEXLETOL did not include patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) or patients with ESRD on dialysis [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The pharmacokinetics of bempedoic acid and its metabolite (ESP15228) was studied in patients with normal hepatic function or mild or moderate hepatic impairment (Child-Pugh A or B) following a single dose (n = 8/group). Compared to patients with normal hepatic function, the bempedoic acid mean Cmax and AUC were decreased by 11% and 22%, respectively, in patients with mild hepatic impairment and by 14% and 16%, respectively, in patients with moderate hepatic impairment. Compared to patients with normal hepatic function, the ESP15228 mean Cmax and AUC were decreased by 13% and 23%, respectively, in patients with mild hepatic impairment and by 24% and 36%, respectively, in patients with moderate hepatic impairment. This is not expected to result in lower efficacy.
Bempedoic acid was not studied in patients with severe hepatic impairment (Child Pugh C) [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Cytochrome P450 Substrates
In vitro metabolic interaction studies suggest that bempedoic acid, as well as its active metabolite and glucuronide forms are not metabolized by and do not interact with cytochrome P450 enzymes.
Transporter-mediated Drug Interactions
In vitro drug interaction studies suggest bempedoic acid, as well as its active metabolite and glucuronide form, are not substrates of commonly characterized drug transporters with the exception of bempedoic acid glucuronide, which is an OAT3 substrate. Bempedoic acid weakly inhibits OAT3 at high multiples of clinically relevant concentrations, and bempedoic acid and its glucuronide weakly inhibit OATP1B1, and OATP1B3 at clinically relevant concentrations. Bempedoic acid weakly inhibits OAT2 in vitro, which is likely the mechanism responsible for minor elevations in serum creatinine and uric acid [see Adverse Reactions (6.1)].
Probenecid
Administration of bempedoic acid 180 mg with steady-state probenecid resulted in a 1.7- and a 1.2-fold increase in bempedoic acid AUC and Cmax, respectively. AUC and Cmax for bempedoic acid active metabolite (ESP15228) were increased 1.9- and 1.5-fold, respectively. These elevations are not clinically meaningful and do not impact dosing recommendations.
Statins
The pharmacokinetic interactions between bempedoic acid (at systemic exposure relevant to the indicated ASCVD population) and simvastatin 20 mg, atorvastatin 10 mg, pravastatin 40 mg, and rosuvastatin10 mg were evaluated in clinical trials.
Simvastatin: Administration of simvastatin 20 mg with 240 mg of bempedoic acid or 40 mg with 180 mg of bempedoic acid in healthy subjects at steady-state resulted in approximately 2-fold (91% for 20 mg and 96% for 40 mg) and 1.5-fold (54% for 20 mg and 52% for 40 mg) increases in simvastatin acid AUC and Cmax, respectively [See Drug Interactions (7)].
Pravastatin: Administration of pravastatin 40 mg with steady-state bempedoic acid 240 mg in healthy subjects resulted in 99% (2-fold) and 104% (2-fold) increases in pravastatin acid AUC and Cmax, respectively [see Drug Interactions (7)].
Ezetimibe
Increases in AUC and Cmax for ezetimibe were less than 20% when a single dose of ezetimibe was taken with steady-state bempedoic acid. Total ezetimibe (ezetimibe and its glucuronide form) and ezetimibe glucuronide AUC and Cmax increased approximately 1.6- and 1.8-fold, respectively. These elevations are not clinically meaningful and do not impact dosing recommendations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Bempedoic acid was negative for mutagenicity in an in vitro Ames assay and negative for clastogenicity in the vitro human lymphocyte chromosome aberration assay. Bempedoic acid was negative in both in vivo mouse micronucleus and in vivo rat bone marrow micronucleus/liver comet assay. In a 2-year rat carcinogenicity study, Wistar rats were given oral doses of bempedoic acid at 3, 10 and 30 mg/kg/day. An increased incidence of liver hepatocellular adenomas and hepatocellular adenomas combined with carcinomas, thyroid gland follicular cell adenoma and follicular cell adenomas combined with carcinomas, and pancreatic islet cell adenomas combined with carcinomas were observed in male rats at the dose of 30 mg/kg/day (exposure equivalent to the maximum recommended human dose (MRHD), based on AUC). In a 2-year mice carcinogenicity study, CD-1 mice were given oral doses of bempedoic acid at 25, 75 and 150 mg/kg/day. Bempedoic acid-related increases in the incidence of liver hepatocellular adenomas, hepatocellular carcinomas and hepatocellular adenomas combined with carcinomas in male mice were observed at 75 and 150 mg/kg/day (exposures equivalent to the MRHD). Observations of liver and thyroid tumors are consistent with PPAR alpha agonism in rodents. The human relevance of pancreatic islet cell tumor findings is unknown.
In fertility and early embryofetal development study in rats, bempedoic acid was given orally to male and female rats at 10, 30 and 60 mg/kg/day. Males were dosed for 28 days prior to mating and females were dosed 14 days prior to mating through gestation day 7. No adverse effects on fertility were observed in females in the absence of maternal toxicity. No effects were observed on male fertility outcomes, but decreases in sperm counts were observed at 60 mg/kg/day (9 times the MRHD).
-
14 CLINICAL STUDIES
The efficacy of NEXLETOL was investigated in two multi-center, randomized, double-blind, placebo-controlled trials that enrolled 3009 adult patients with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who were on maximally tolerated statin therapy. Demographics and baseline disease characteristics were balanced between the treatment arms in all trials. In both trials, the maximum LDL-C lowering effects occurred at Week 4. These results were consistent across all subgroups studied in any of the trials, including age, gender, race, ethnicity, region, history of diabetes, baseline LDL-C, body mass index (BMI), HeFH status, and background therapies.
Study 1 (NCT02666664)
Study 1 was a multi-center, randomized, double-blind, placebo-controlled 52-week trial that evaluated safety and efficacy of bempedoic acid in patients with HeFH and/or ASCVD. Efficacy of NEXLETOL was evaluated at Week 12. The trial included 2230 patients randomized 2:1 to receive either NEXLETOL (n = 1488) or placebo (n = 742) as add-on to a maximally tolerated lipid lowering therapy. Maximally tolerated lipid lowering therapy was defined as a maximally tolerated statin dose alone or in combination with other lipid-lowering therapies. Patients were stratified by presence of HeFH and by baseline statin intensity. Patients on simvastatin 40 mg per day or higher and patients taking PCSK9 inhibitors were excluded from the trial.
Overall, the mean age at baseline was 66 years (range: 24 to 88 years), 61% were ≥ 65 years old, 27% women, 2% Hispanic, 96% White, 3% were Black, and 1% Asian. Ninety-five percent (95%) of patients had established atherosclerotic cardiovascular disease, and 5% of patients had HeFH. Twenty-nine percent (29%) of patients had diabetes at baseline. The mean baseline LDL-C was 103.2 mg/dL. At the time of randomization, all patients were receiving statin therapy and 50% were receiving high-intensity statin therapy.
The primary efficacy outcome measure of the study was the percent change from baseline to Week 12 in LDL-C. The difference between NEXLETOL and placebo in mean percent change in LDL-C from baseline to Week 12 was -18% (95% CI: -20%, -16%; p < 0.001). High-density lipoprotein (HDL) and triglycerides (TG) were examined as exploratory endpoints and were not included in the statistical hierarchy. The difference between NEXLETOL and placebo in mean percent change from baseline to Week 12 was -6% for HDL and median percent change from baseline to Week 12 was +3% for TG. For additional results see Table 2 and Figure 1.
Table 2: Effects of NEXLETOL on Lipid Parameters in Patients with HeFH and/or ASCVD on Maximally Tolerated Statin Therapy (Mean % Change from Baseline to Week 12 in Study 1) LDL-C*,† Non-HDL-C† apo B† TC† apo B = apolipoprotein B; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol. Background statin: atorvastatin, simvastatin, pravastatin, - * 4.3% of subjects on NEXLETOL and 2.3% of subjects on placebo had missing LDL-C data at primary endpoint (Week 12). By the end of the trial (Week 52), 8.3% of subjects on NEXLETOL and 7.7% of subjects on placebo had missing LDL-C measurements.
- † Percent change from baseline was analyzed using analysis of covariance (ANCOVA), with treatment and randomization strata (HeFH versus ASCVD, and high intensity statin versus other statin) as factors and baseline lipid parameter as a covariate. Missing data for LDL-C, non-HDL-C, TC and apo B were imputed through multiple imputation using a pattern mixture model (PMM) account for treatment adherence.
- ‡ Number of randomized subjects at baseline
NEXLETOL ± Statin ± Other Lipid Lowering Therapies
(180 mg/day; n = 1488‡)-17 -12 -9 -10 Placebo
(n = 742‡)2 2 3 1 Mean Difference from Placebo (95% CI) -18
(-20, -16)-13
(-15, -12)-12
(-14, -10)-11
(-13, -10)Study 2 (NCT02991118)
Study 2 was a multi-center, randomized, double-blind, placebo-controlled, 52-week trial in patients with HeFH and/or ASCVD. Efficacy of NEXLETOL was evaluated at Week 12. The trial included 779 patients randomized 2:1 to receive either NEXLETOL (n = 522) or placebo (n = 257) as add-on to a maximally tolerated lipid lowering therapy. Maximally tolerated lipid lowering therapy was defined as a maximally tolerated statin dose alone or in combination with other lipid-lowering therapies. Patients were stratified by presence of HeFH and baseline statin intensity. Patients on simvastatin 40 mg/day or higher were excluded from the trial.
Overall, the mean age at baseline was 64 years (range: 28 to 91 years), 51% were ≥ 65 years old, 36% women, 8% Hispanic, 94% White, 5% were Black, and 1% Asian. Ninety-five percent (95%) of patients had established atherosclerotic cardiovascular disease, and 5% of patients had HeFH. Thirty percent (30%) of patients had diabetes at baseline. The mean baseline LDL-C was 120.4 mg/dL. At the time of randomization, 90% of patients were receiving statin therapy, 53% were receiving high-intensity statin therapy, and 0.3% were receiving PCSK9 inhibitors.
The primary efficacy outcome measure of the study was the percent change from baseline to Week 12 in LDL-C. The difference between NEXLETOL and placebo in mean percent change in LDL-C from baseline to Week 12 was -17 % (95% CI: -21%, -14%; p < 0.001). HDL and TG were exploratory endpoints and not included in the statistical hierarchy. The difference between NEXLETOL and placebo in mean percent change from baseline to Week 12 was -6% for HDL and the median percent change from baseline was -2% for TG. For additional results see Table 3 and Figure 1.
Table 3. Effects of NEXLETOL on Lipid Parameters in Patients with HeFH and/or ASCVD on Maximally Tolerated Statin Therapy (Mean % Change from Baseline to Week 12 in Study 2) LDL-C*,† Non-HDL-C† apo B† TC† apo B = apolipoprotein B; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol. Background statin: atorvastatin, simvastatin, rosuvastatin, pravastatin, fluvastatin, pitavastatin, and lovastatin. - * 4.6% of subjects on NEXLETOL and 1.6% of subjects on placebo had missing LDL-C data at primary endpoint (Week 12). By the end of the trial (Week 52), 10.5% of subjects on NEXLETOL and 7.8% of subjects on placebo had missing LDL-C measurements.
- † Percent change from baseline was analyzed using analysis of covariance (ANCOVA), with treatment and randomization strata (HeFH versus ASCVD, and high intensity statin versus other statin) as factors and baseline lipid parameter as a covariate. Missing data for LDL-C, non-HDL-C, TC and apo B were imputed through multiple imputation using a pattern mixture model (PMM) account for treatment adherence.
- ‡ Number of randomized subjects at baseline
NEXLETOL ± Statin ± Other Lipid Lowering Therapies
(180 mg/day; n = 522‡)-15 -11 -9 -10 Placebo
(n = 257‡)2 2 4 1 Difference from Placebo (95% CI) -17
(-21, -14)-13
(-16, -10)-13
(-16, -10)-11
(-14, -9)Figure 1: Mean Percent Change from Baseline in LDL-C Over 52 Weeks in Patients with HeFH and/or ASCVD on Maximally Tolerated Statin Treated with NEXLETOL and Placebo (Study 1 and Study 2)
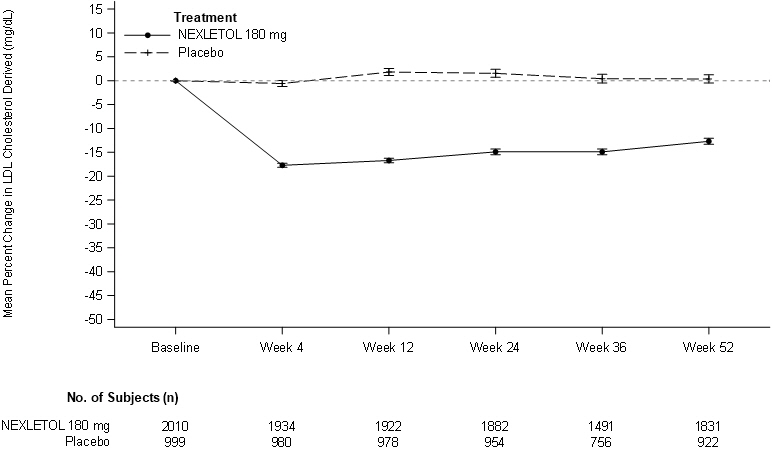
LDL-C derived is calculated from the Friedewald equation: LDL-C = TC - HDL-C - TG/5 in mg/dL.
The error bars represent standard error. -
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
NEXLETOL (bempedoic acid) tablets are supplied as follows:
Tablet Strength Description Package Configuration NDC No. 180 mg White to off white and oval, debossed with "180" on one side and "ESP" on the other side Bottle of 30 tablets with child-resistant cap 72426-118-03 Bottle of 90 tablets with child-resistant cap 72426-118-09 -
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling.
Risk of Hyperuricemia
Advise patients of the risk of elevated serum uric acid levels, including development of gout. Inform patients that serum uric acid levels may be monitored during treatment with NEXLETOL. Patients with signs or symptoms of hyperuricemia should contact their healthcare provider if symptoms occur [See Warnings and Precautions (5.1)]
Risk of Tendon Rupture
Inform patients of the risk of tendon rupture. Advise patients to rest at the first sign of tendinitis or tendon rupture and to immediately contact their healthcare provider if tendinitis or tendon rupture symptoms occur [see Warnings and Precautions (5.2)].
Risk of Myopathy with Concomitant Use of Simvastatin or Pravastatin
Advise patients to notify their healthcare provider(s) if they are taking, or plan to take simvastatin or pravastatin. The risk of myopathy occurring with the use of simvastatin or pravastatin may be increased when taken with NEXLETOL. [see Drug Interactions (7)].
Pregnancy
Advise pregnant women of the potential risk to a fetus based on NEXLETOL's mechanism of action. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
NEXLETOL™ (NEX-le-tol)
(bempedoic acid)
tablets, for oral useThis Patient Package Insert has been approved by the U.S. Food and Drug Administration Revised: 02/2020 What is NEXLETOL?
NEXLETOL is a prescription medicine used along with diet and other lipid-lowering medicines in the treatment of adults with:- heterozygous familial hypercholesterolemia (HeFH). HeFH is an inherited condition that causes high levels of "bad" cholesterol called low density lipoprotein (LDL).
- known heart disease who need additional lowering of "bad" cholesterol (LDL-C) levels.
It is not known if NEXLETOL is safe and effective in people with severe kidney problems including people with end-stage kidney disease who are on dialysis.
It is not known if NEXLETOL is safe and effective in people with severe liver problems.
It is not known if NEXLETOL is safe and effective in children under 18 years of age.Before you start taking NEXLETOL, tell your healthcare provider about all your medical conditions, including if you: - have or had gout.
- have or had tendon problems.
- are pregnant. Tell your healthcare provider right away if you become pregnant while taking NEXLETOL. You and your healthcare provider will decide if you should take NEXLETOL while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if NEXLETOL passes into your breast milk. You and your healthcare provider should decide if you will take NEXLETOL or breastfeed. You should not do both.
- have severe kidney problems.
- have severe liver problems.
Especially tell your healthcare provider if you take or plan to take simvastatin or pravastatin (other cholesterol lowering medicines). Taking simvastatin or pravastatin with NEXLETOL may increase your risk of developing muscle pain or weakness (myopathy).Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I take NEXLETOL? - Take NEXLETOL exactly as your healthcare provider tells you to take it. Check with your healthcare provider or pharmacist if you are not sure.
- Take 1 NEXLETOL tablet by mouth each day.
- You may take NEXLETOL with or without food.
- If you take too much NEXLETOL, call your poison control center at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are possible side effects of NEXLETOL?
NEXLETOL may cause serious side effects, including:- increased levels of uric acid in your blood (hyperuricemia). This can happen within 4 weeks of you starting NEXLETOL and continue throughout your treatment. Your healthcare provider may monitor your blood uric acid levels while you are taking NEXLETOL. High levels of blood uric acid may lead to gout. Call your healthcare provider if you have the following symptoms of hyperuricemia and gout:
- severe foot pain especially in the toe joint
- warm joints
- swelling
- tender joints
- joint redness
Gout may happen more in people who have had gout before but also can happen in people who have never had it before. -
tendon rupture or injury. Tendon problems can happen in people who take NEXLETOL. Tendons are tough cords of tissue that connect muscles to bones. Symptoms of tendon problems may include pain, swelling, tears, and inflammation of tendons including the arm, shoulder, and back of the ankle (Achilles).
- Tendon rupture can happen while you are taking NEXLETOL. Tendon ruptures can happen within weeks or months of starting NEXLETOL.
- The risk of getting tendon problems while you take NEXLETOL is higher if you:
- are over 60 years of age
- are taking antibiotics (fluoroquinolones)
- have had tendon problems
- are taking steroids (corticosteroids)
- have renal failure
-
Stop taking NEXLETOL immediately and get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
- hear or feel a snap or pop in a tendon area
- bruising right after an injury in a tendon area
- unable to move the affected area or put weight on the affected area
- Talk to your healthcare provider about the risk of tendon rupture with continued use of NEXLETOL. You may need a different lipid-lowering medicine to treat your cholesterol levels.
- symptoms of the common cold, flu, or flu-like symptoms
- back pain
- stomach pain
- increased liver enzymes
- muscle spasms
- pain in shoulder, legs, or arms
- anemia
- bronchitis
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of NEXLETOL.
For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store NEXLETOL? - Store NEXLETOL in the original package at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not throw away the packet that helps to keep your medicine dry (desiccant).
General information about the safe and effective use of NEXLETOL.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NEXLETOL for a condition for which it was not prescribed. Do not give NEXLETOL to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about NEXLETOL that is written for healthcare professionals.What are the ingredients in NEXLETOL? - active ingredient: bempedoic acid
- inactive ingredients: colloidal silicon dioxide, hydroxyl propyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate
- tablet coating: partially hydrolyzed polyvinyl alcohol, polyethylene glycol, talc, and titanium dioxide
Manufactured by:
Piramal Healthcare UK Limited
Northumberland NE61 3YA
United Kingdom
Manufactured for:
Esperion Therapeutics, Inc.
3891 Ranchero Drive, Suite 150
Ann Arbor, MI 48108
© 2020 Esperion Therapeutics, Inc. - PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
NEXLETOL
bempedoic acid tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72426-118 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bempedoic Acid (UNII: 1EJ6Z6Q368) (Bempedoic Acid - UNII:1EJ6Z6Q368) Bempedoic Acid 180 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE (White to off-white) Score no score Shape OVAL Size 14mm Flavor Imprint Code 180;ESP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72426-118-03 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/06/2020 2 NDC: 72426-118-09 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/06/2020 3 NDC: 72426-118-99 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 03/06/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211616 03/06/2020 Labeler - Esperion Therapeutics, Inc. (029516312) Establishment Name Address ID/FEI Business Operations Piramal Healthcare UK Limited 345609965 MANUFACTURE(72426-118) , ANALYSIS(72426-118) , PACK(72426-118) , LABEL(72426-118) Establishment Name Address ID/FEI Business Operations Anderson Brecon, Inc. DBA Packaging Coordinators, Inc. (PCI) 053217022 PACK(72426-118) , LABEL(72426-118) Establishment Name Address ID/FEI Business Operations Packaging Coordinators, LLC DBA Packaging Coordinators, Inc. (PCI) 078525133 PACK(72426-118) , LABEL(72426-118)
Trademark Results [Nexletol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEXLETOL 87847415 not registered Live/Pending |
Esperion Therapeutics, Inc. 2018-03-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.