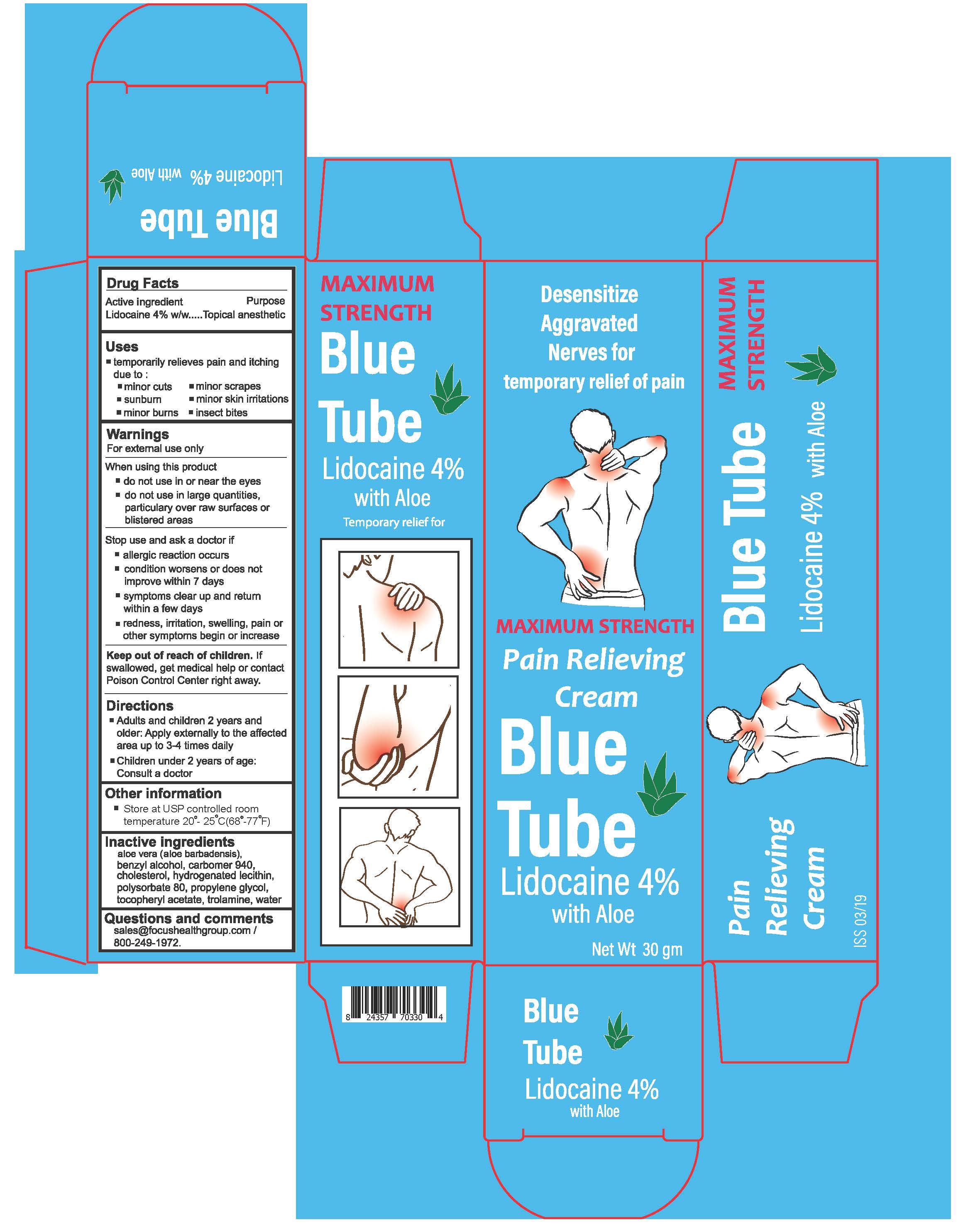
BLUE TUBE- lidocaine 4% cream
Blue Tube by
Drug Labeling and Warnings
Blue Tube by is a Otc medication manufactured, distributed, or labeled by Focus Health Group, DSC Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient:
- Purpose
- Uses
-
WARNINGS
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
- Directions
- Other Information
- Inactive ingredients
- Questions and comments
- Blue Tube Lidocaine 4% 30g Tube
-
INGREDIENTS AND APPEARANCE
BLUE TUBE
lidocaine 4% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 24357-703 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) CHOLESTEROL (UNII: 97C5T2UQ7J) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24357-703-30 1 in 1 CARTON 05/16/2019 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/16/2019 Labeler - Focus Health Group (826939949) Registrant - DSC Laboratories Inc. (097807374) Establishment Name Address ID/FEI Business Operations DSC Laboratories Inc. 097807374 manufacture(24357-703) Establishment Name Address ID/FEI Business Operations Focus Health Group 826939949 label(24357-703)
Trademark Results [Blue Tube]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BLUE TUBE 77263331 3474239 Dead/Cancelled |
Newtech Associates 2007-08-24 |
 BLUE TUBE 76167395 2760671 Dead/Cancelled |
WINCOR NIXDORF INTERNATIONAL GMBH 2000-11-16 |
 BLUE TUBE 75930070 not registered Dead/Abandoned |
Presonus Audio Electronics, Inc. 2000-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.