BENZEPRO- benzoyl peroxide aerosol, foam
BenzePrO by
Drug Labeling and Warnings
BenzePrO by is a Otc medication manufactured, distributed, or labeled by PruGen, Inc., PHARMASOL CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration
- avoid unnecessary sun exposure and use a sunscreen
-
Directions
See package insert for full prescribing information
Prime can before initial use: See package insert Before Each Use: Shake vigorously
During Use: Holding can upright, dispense into palm of hand and apply to affected area as directed by physician.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Other Information
-
Inactive Ingredients
BHT, C12-15 alkyl benzoate, cetostearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, povidone, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10, stearic acid, trolamine. Also contains: Propellant HFA-134A (1, 1, 1, 2-tetrafluoroethane).
Questions? 866-696-8525
Manufactured for:
PruGen, Inc.
Pharmaceuticals
18899 North Thompson Peak Parkway
Scottsdale, Arizona 85255 REV 1.2 -
PRINCIPAL DISPLAY PANEL - 100 g Can Box
NDC: 42546-010-10
Rx onlyBenzePrO®
Emollient Foam
benzoyl peroxide
5.3%For topical treatment
of mild to moderate
acne vulgarisNet Weight 100 g
Will not dispense entire contents.
Container is overfilled to guarantee
dispensing at least the listed amount.PRUGEN™
PHARMACEUTICALS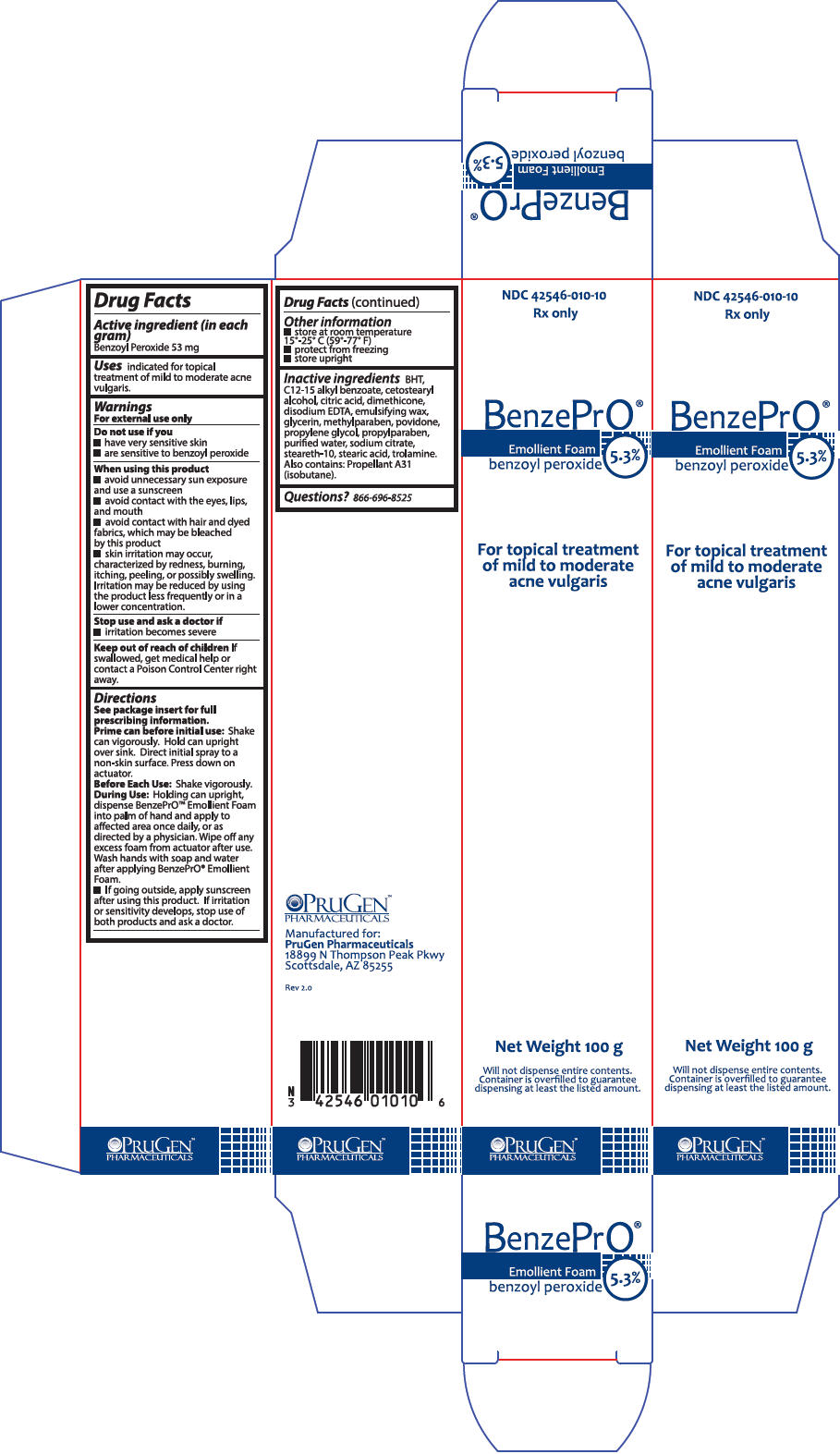
-
INGREDIENTS AND APPEARANCE
BENZEPRO
benzoyl peroxide aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42546-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 3.18 g in 60 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) STEARETH-10 (UNII: FD0913P475) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42546-010-10 1 in 1 BOX 07/01/2012 1 100 g in 1 CAN; Type 0: Not a Combination Product 2 NDC: 42546-010-01 8 in 1 CARTON 07/01/2012 2 5 g in 1 CANISTER; Type 0: Not a Combination Product 3 NDC: 42546-010-06 1 in 1 BOX 07/01/2012 3 60 g in 1 CANISTER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 07/01/2012 Labeler - PruGen, Inc. (929922750) Establishment Name Address ID/FEI Business Operations PHARMASOL CORPORATION 065144289 MANUFACTURE(42546-010)
Trademark Results [BenzePrO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BENZEPRO 85765828 4564957 Live/Registered |
PRUGEN, LLC 2012-10-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.