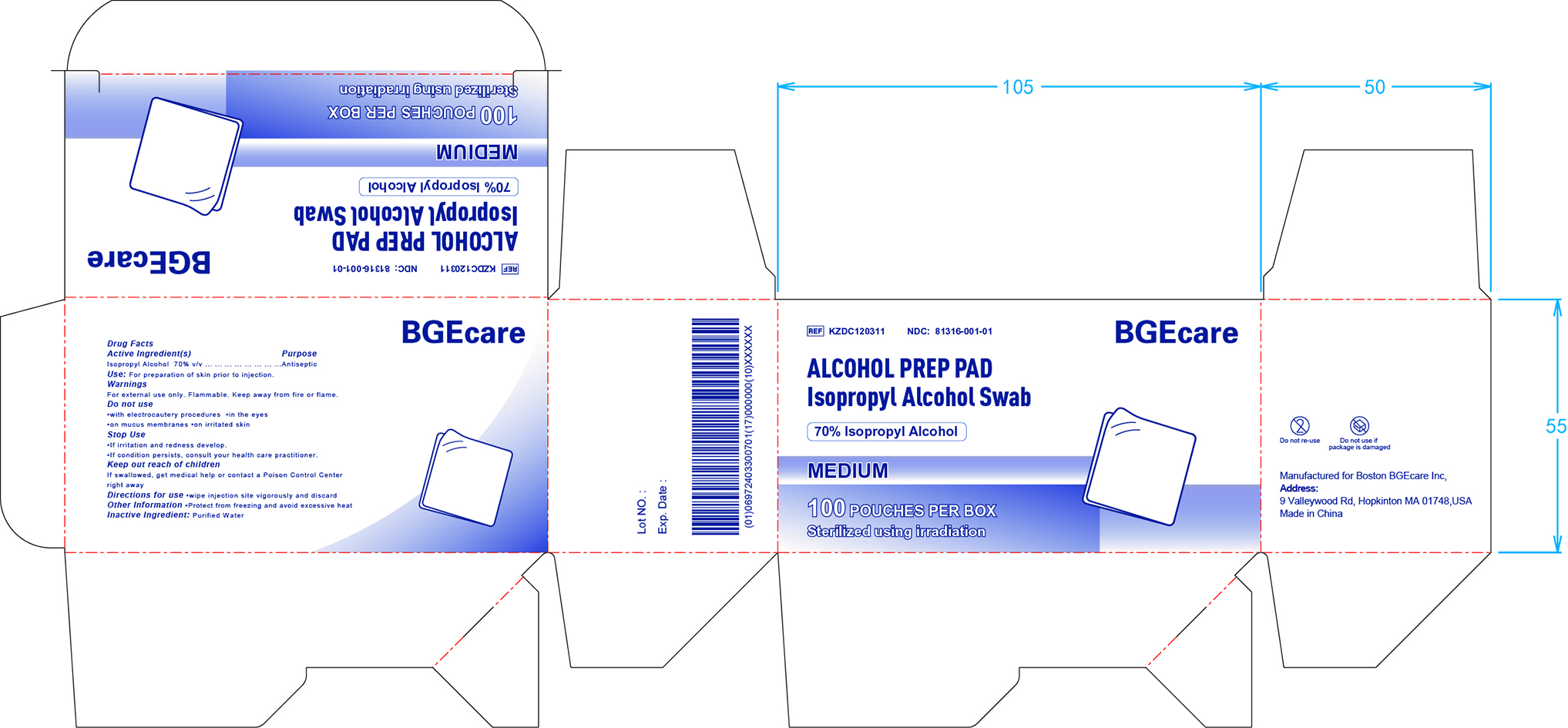
81316-001 Alcohol Prep Pad
BGEcare ALCOHOL PREP PAD Isopropyl Alcohol Swab by
Drug Labeling and Warnings
BGEcare ALCOHOL PREP PAD Isopropyl Alcohol Swab by is a Otc medication manufactured, distributed, or labeled by BOSTON BGECARE INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BGECARE ALCOHOL PREP PAD ISOPROPYL ALCOHOL SWAB- isopropyl alcohol solution
BOSTON BGECARE INC
----------
81316-001 Alcohol Prep Pad
Do not use:
- with electrocautery procesures
- in the eyes
- on mucus membranes
- on irritated skin
Stop use:
- if irritation and redness develop
- if condition persists, consult your health care practitioner
| BGECARE ALCOHOL PREP PAD ISOPROPYL ALCOHOL SWAB
isopropyl alcohol solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BOSTON BGECARE INC (056757726) |
Revised: 1/2024
Document Id: 0e7b40c9-7575-3b5b-e063-6394a90a3815
Set id: 894ec218-215a-4e76-8996-87af0920840d
Version: 5
Effective Time: 20240108
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.