4346 First Aid Kit by Honeywell Safety Products USA, INC 4346 FIRST AID KIT kit
4346 First Aid Kit by
Drug Labeling and Warnings
4346 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Eyewash Active ingredient
- Eyewash Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyewash Directions
- Eyewash Inactive ingredients
- Eyewash Questions
- Triple Active ingredients
- Triple Purpose
- Triple Uses
- Triple Warnings
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
- Miralac Active ingredient (in each chewable tablet)
- Miralac Purpose
- Miralac Uses
-
Warnings
Ask a doctor before use if you have
- kidney stones
- calcium-restricted diet
Ask a doctor before use if you are
- presently taking a prescription drug. Antacids may interfere with certain prescription drugs
- Miralac Directions
- Miralac Other information
- Miralac Inactive ingredients
- Miralac Questions or comments?
- BZK Active ingredient
- BZK Purpose
- BZK Uses
-
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Directions
- BZK Other information
- BZK Inactiave ingredient
- BzK Questions
- Questions or Comments?
- Pain Stopper Active ingredient (in each tablet)
- Pain Stopper Purpose
- Pain Stopper Uses
-
Pain Stopper
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you are taking a diuretic
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
Stop using and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pin that does not get better
- if ringing in the ears or a loss of hearing occurs, consult a doctor before taking any more of this product.
- Pain Stopper Directions
- Pain Stopper Other information
- Pain Stopper Inactive ingredients
- Pain Stopper Questions or Comments?
- Burn relief WJ Active ingredient
- Burn Relief WJ Purpose
- Burn Relief WJ Uses
- Burn Relief WJ Warnings
- Burn Relief WJ Directions
- Burn Relief WJ Other information
- Burn Relief WJ Inactive ingredients
- Burn Relief WJ Questions or Comments?
-
4346
Z68140GRR KIT CONTENTS
1 1X3 PLASTIC 100/BOX
1 WOVEN 7/8 X 3 50/BOX
1 SWIFT KNUCKLE 40/BX
1 SWIFT FINGERTIP 8 50/BOX
2 TRIPLE ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 INSTANT COLD PACK 4" X 6"
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 BLOODSTOPPER
1 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
2 ANTISEPTIC WIPES BZK CHL 20'S
1 PAIN STOPPERS IND PK 2ENV 100
1 MIRALAC TABS IND PK 2/ENV 100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 F A KIT EMPTY BLANK 140
1 POCKET INSERT RED #140 KIT 2R
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
1 TRI BNDG NON WOVEN 40"X40"X56"
- Eyewash Principal Display Panel
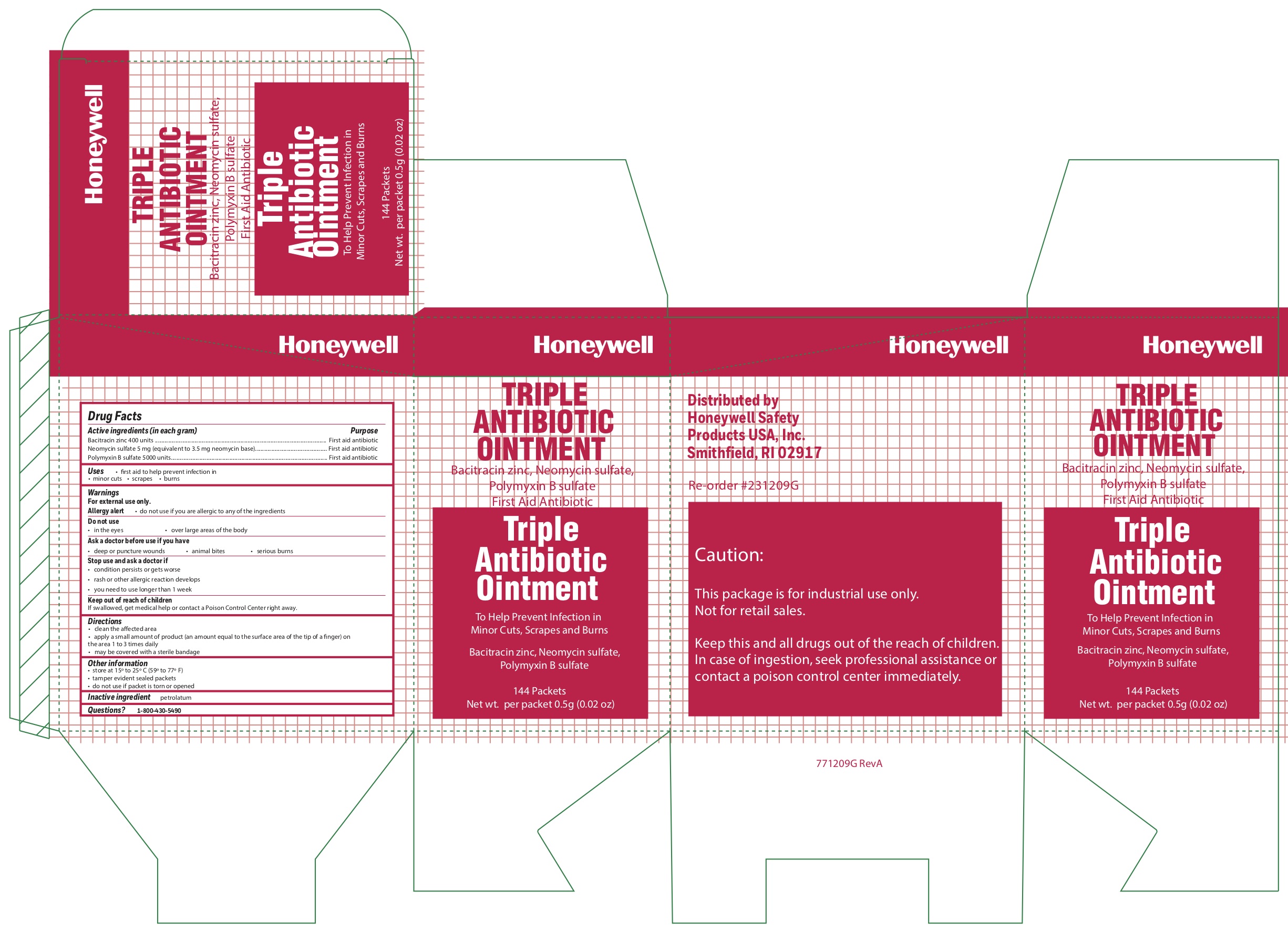
- Triple Principal Display Panel
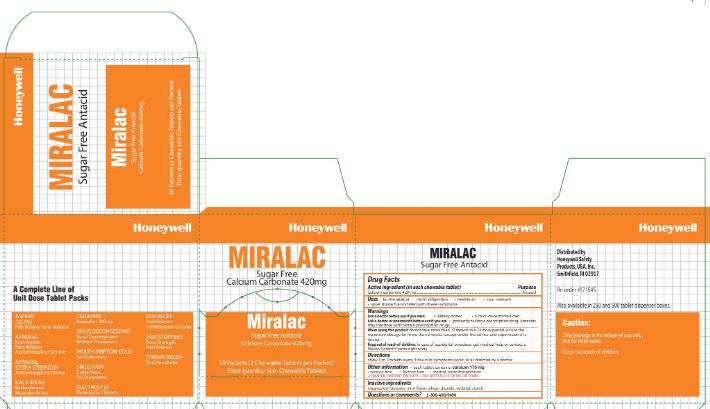
- Miralac Principal Display Panel
- BZK Principal Display Panel
- Pain Stopper Principal Display Panel
- Burn Relieff WJ Principal Display Panel
- 4346 Kit Label Z68140GRR
-
INGREDIENTS AND APPEARANCE
4346 FIRST AID KIT
4346 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4346 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4346-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 118 mL Part 2 1 BOTTLE 59 mL Part 3 20 PACKET 18 g Part 4 50 PACKET 100 Part 5 40 PACKET 56 mL Part 6 50 PACKET 100 Part 1 of 6 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 2 of 6 BURN RELIEF
lidocaine hydrochloride sprayProduct Information Item Code (Source) NDC: 0498-0221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 24.64 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) HYPROMELLOSES (UNII: 3NXW29V3WO) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) WATER (UNII: 059QF0KO0R) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0221-59 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/18/2018 Part 3 of 6 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC: 0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 4 of 6 MIRALAC
calcium carbonate tabletProduct Information Item Code (Source) NDC: 0498-0303 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 420 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score 2 pieces Shape ROUND Size 11mm Flavor MINT Imprint Code FR8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 02/22/2012 Part 5 of 6 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/22/2017 Part 6 of 6 PAIN STOPPERS
acetaminophen, caffeine, aspirin, salicylamide tabletProduct Information Item Code (Source) NDC: 0498-2422 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 110 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 32.4 mg SALICYLAMIDE (UNII: EM8BM710ZC) (SALICYLAMIDE - UNII:EM8BM710ZC) SALICYLAMIDE 152 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 162 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE K30 (UNII: U725QWY32X) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange (BRIGHT ORANGE) Score no score Shape ROUND Size 11mm Flavor Imprint Code FR;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-2422-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 01/02/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4346) Establishment Name Address ID/FEI Business Operations Ultra Seal Corporation 085752004 manufacture(0498-2422, 0498-0303) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0750, 0498-0221) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0501)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.