OXERVATE- cenegermin-bkbj kit OXERVATE- cenegermin-bkbj solution/ drops
OXERVATE by
Drug Labeling and Warnings
OXERVATE by is a Prescription medication manufactured, distributed, or labeled by Dompé farmaceutici S.p.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OXERVATE safely and effectively. See full prescribing information for OXERVATE.
OXERVATETM (cenegermin-bkbj) ophthalmic solution, for topical ophthalmic use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
OXERVATE is a recombinant human nerve growth factor indicated for the treatment of neurotrophic keratitis. (1)
DOSAGE AND ADMINISTRATION
One drop of OXERVATE in the affected eye(s), 6 times per day at 2-hour intervals, for eight weeks. (2.1)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: cenegermin-bkbj 0.002% (20 mcg/mL) in a multiple-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Patients should remove contact lenses before applying OXERVATE and wait 15 minutes after instillation of the dose before reinsertion. (5.1)
ADVERSE REACTIONS
The most common adverse reactions (incidence >5%) are eye pain, ocular hyperemia, eye inflammation and increased lacrimation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Dompé U.S. Inc. at 1-833-366-7387 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Recommended Dosage and Dose Administration
2.3 Preparation for Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Use with Contact Lens
5.2 Eye Discomfort
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
Contact lenses should be removed before applying OXERVATE and may be reinserted 15 minutes after administration.
If a dose is missed, treatment should be continued as normal, at the next scheduled administration.
If more than one topical ophthalmic product is being used, administer the eye drops at least 15 minutes apart to avoid diluting products. Administer OXERVATE 15 minutes prior to using any eye ointment, gel or other viscous eye drops.
2.2 Recommended Dosage and Dose Administration
Instill one drop of OXERVATE in the affected eye(s), 6 times a day at 2-hour intervals for eight weeks.
2.3 Preparation for Administration
Remove the weekly cartons of OXERVATE from the insulated container and store it for up to 14 days in a refrigerator (no later than 5 hours from when you receive the medicine from your pharmacy). OXERVATE is stored in a freezer at the pharmacy. If treatment is started immediately after receiving the weekly carton, wait until the first vial is thawed (this could take up to 30 minutes when kept at room temperature up to 77°F (25°C)). Do not shake the vial.
Follow Steps 1 to 19 each day you use OXERVATE:
Take an individual vial of OXERVATE from the refrigerator in the morning and prepare it in the following way:
Step 1. Wash your hands.
Step 2. If you wear contact lenses, take them out before using OXERVATE.
Step 3. Remove the plastic flip-off cap from the vial.
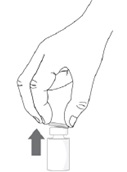
Step 4. Peel-off the back of the vial adapter blister pack.
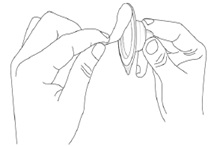
Step 5. Without removing the vial adapter from its blister pack, connect it to the vial by firmly pushing it down until it snaps into place over the neck of the vial. The spike of the vial adapter should pierce through the vial’s rubber stopper. After the vial adapter has been connected correctly, do not remove it from the vial.
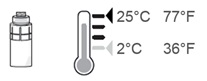
Note: After the vial adapter is connected to the vial, OXERVATE can be stored in the refrigerator between 36°F to 46°F (2°C to 8°C) for up to 12 hours.
If needed, the OXERVATE with the connected vial adapter may be stored at room temperature up to 77°F (25°C).
Step 6. Remove and throw away the packaging of the vial adapter.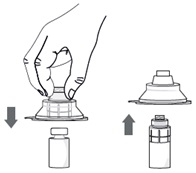
The multi-dose vial of OXERVATE is now ready for use (1 drop in the affected eye every 2 hours six times a day).
To withdraw and give each dose of OXERVATE, follow the Steps 7 to 19:
Step 7. Take a single sterile disinfectant wipe and gently clean the surface of the valve on the connector part of the vial adapter.
After cleaning, wait for about 1 minute to allow the valve to dry.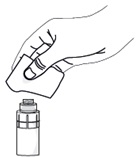
Step 8. Remove a pipette from its protective packaging.
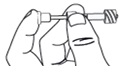
Step 9. Screw the pipette (clockwise) into the connector part of the vial adapter.
Step 10. Make sure that the pipette plunger is pushed all the way down.
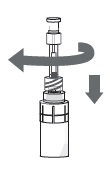
Step 11. Turn the vial upside-down with the pipette still connected. Gently pull the plunger until it stops, to draw the eye drop solution into the pipette. Make sure the plunger has reached the stop point.
Step 12. Check the pipette to make sure it contains the eye drop solution. Air bubbles may cause blockage and prevent the pipette from filling properly (especially the first time you withdraw the eye drop solution). If the pipette is empty, keep the vial with the connected pipette upside-down, push the plunger all the way in and pull it out again.
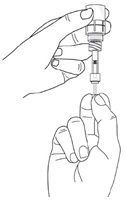
Step 13. After the pipette has been correctly filled, unscrew the pipette from the connector part of the vial adapter (counter-clockwise). Pull the pipette straight up to remove it.
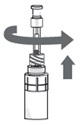
Step 14.
- Sit or lie down to steady yourself when you instill OXERVATE.
- Holding the pipette, pointing down, between your middle finger and thumb, tilt your head back and position the pipette above your affected eye.
- With your other hand, pull down your lower eyelid, increasing the space between the inner eyelid and the eyeball (the conjunctival fornix).
- Gently push the plunger down until at least a drop is released into the conjunctival fornix.
- Make sure you do not touch your eye with the tip of the pipette.
- With your head still tilted back, blink a few times so that the medicine covers the surface of your eye.
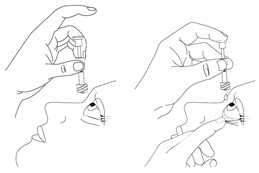
Step 15. Throw away the used pipette right away after use, even if there is still some eye drop solution left in it.
If you miss your eye and there is no longer eye drop solution in the pipette, try again, using a new pipette and wipe (See Steps 7 to 14).
Step 16. After each use throughout the day, place the vial back in the refrigerator or keep it below 77°F (25°C) for the rest of the day, with the vial adapter still connected.
Step 17. Repeat from Step 7 to Step 16 every 2 hours 6 times a day, using a new sterile disinfectant wipe and a new pipette each time.
If you use drops in both eyes, repeat the above instructions for your other eye using a new pipette. You will need to use 2 vials each day.
Store the vial below 77°F (25°C) throughout the day. You can also store the vial in the refrigerator but do not freeze the vial.
Step 18. Throw away the used vial at the end of each day even if there is still some eye drop solution left in it. Throw away the vial no later than 12 hours from the time you connected the vial adapter to it.
Step 19. Track each time you instill an eye drop of OXERVATE on the weekly Dose Recording Card provided with the delivery system.
This will allow you to track your 6 doses each treatment day, the date of the first use of the weekly supply and the time of the vial opening (which is when you connect the vial adapter to the vial) during the week.
To make sure accurate dosing every 2 hours, you may want to set an alarm as a reminder for dosing.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Use with Contact Lens
Contact lenses should be removed before applying OXERVATE because the presence of a contact lens (either therapeutic or corrective) could theoretically limit the distribution of cenegermin-bkbj onto the area of the corneal lesion. Lenses may be reinserted 15 minutes after administration.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In two clinical trials of patients with neurotrophic keratitis, a total of 101 patients received cenegermin-bkbj eye drops at 20 mcg/mL at a frequency of 6 times daily in the affected eye(s) for a duration of 8 weeks. The mean age of the population was 61 to 65 years of age (18 to 95). The majority of the treated patients were female (61%). The most common adverse reaction was eye pain following instillation which was reported in approximately 16% of patients. Other adverse reactions occurring in 1-10% of OXERVATE patients and more frequently than in the vehicle-treated patients included corneal deposits, foreign body sensation, ocular hyperemia, ocular inflammation and tearing.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data from the use of OXERVATE in pregnant women to inform any drug associated risks.Administration of cenegermin-bkbj to pregnant rats or rabbits during the period of organogenesis did not produce adverse fetal effects at clinically relevant doses. In a pre- and postnatal development study, administration of cenegermin-bkbj to pregnant rats throughout gestation and lactation did not produce adverse effects in offspring at clinically relevant doses.
Data
Animal Data
In embryofetal development studies, daily subcutaneous administration of cenegermin-bkbj to pregnant rats and rabbits throughout the period of organogenesis produced a slight increase in post-implantation loss at doses greater than or equal to 42 mcg/kg/day (267 times the MRHOD). A no observed adverse effect level (NOAEL) was not established for post-implantation loss in either species. In rats, hydrocephaly and ureter anomalies were observed each in one fetuses at 267 mcg/kg/day (1709 times the MRHOD). In rabbits, cardiovascular malformations, including ventricular and atrial septal defects, enlarged heart and aortic arch dilation were observed each in one fetuses at 83 mcg/kg/day (534 times the MRHOD). No fetal malformations were observed in rats and rabbits at doses of 133 mcg/kg/day and 42 mcg/kg/day, respectively.In a pre- and postnatal development study, daily subcutaneous administration of cenegermin-bkbj to pregnant rats during the period of organogenesis and lactation did not affect parturition and was not associated with adverse toxicity in offspring at doses up to 267 mcg/kg/day.
In parental rats and rabbits, an immunogenic response to cenegermin-bkbj was observed. Given that cenegermin-bkbj is a heterologous protein in animals, this response may not be relevant to humans.
8.2 Lactation
Risk Summary
There are no data on the presence of OXERVATE in human milk, the effects on breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for OXERVATE, and any potential adverse effects on the breastfed infant from OXERVATE.8.4 Pediatric Use
The safety and effectiveness of OXERVATE have been established in the pediatric population. Use of OXERVATE in this population is supported by evidence from adequate and well-controlled trials of OXERVATE in adults with additional safety data in pediatric patients from 2 years of age and older [see Clinical Studies (14)].
-
11 DESCRIPTION
OXERVATE ophthalmic solution contains cenegermin-bkbj, a recombinant form of human nerve growth factor produced in Escherichia coli.
Cenegermin-bkbj contains 118 amino acids. Cenegermin-bkbj has a relative molecular mass of 13,266 Daltons and the following molecular formula: C583H908N166O173S8. OXERVATE (cenegermin-bkbj) is a clear, colorless sterile solution with a pH of 7.0-7.4 and osmolarity 280-320 mOsm/kg for topical ophthalmic use.
Each mL contains Active: 20 mcg of cenegermin (0.002% w/v); Inactives: disodium hydrogen phosphate anhydrous (2.87 mg), hydroxypropylmethyl cellulose (1.0 mg), L-methionine (0.01 mg), mannitol (12.22 mg), polyethylene glycol 6000 (10.0 mg), sodium dihydrogen phosphate dihydrate (1.22 mg), trehalose dihydrate (47.03 mg), Water for Injection, USP, and hydrochloric acid and/or sodium hydroxide to adjust pH.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nerve growth factor is an endogenous protein involved in the differentiation and maintenance of neurons, which acts through specific high-affinity (i.e., TrkA) and low-affinity (i.e. p75NTR) nerve growth factor receptors in the anterior segment of the eye to support corneal innervation and integrity.
12.3 Pharmacokinetics
Systemic exposure to cenegermin-bkbj was evaluated by measuring serum nerve growth factor (NGF) concentrations in 20 healthy subjects who received single and multiple (up to six times a day) administration of one drop (35 μL) OXERVATE (0.70 μg of cenegermin-bkbj/ administration). The study also included a placebo arm in 10 healthy subjects who received vehicle only.
At baseline/pre-dose, 17 out of the 20 subjects in the OXERVATE treatment arm had serum NGF concentrations below the limit of assay quantification (LLOQ <15 pg/ml) and the remaining three subjects had serum NGF concentrations ranging from120 pg/ml to 503 pg/ml.
At baseline/pre-dose, 8 of the 10 subjects in the placebo arm had serum NGF concentrations below the limit of assay quantification (LLOQ <15 pg/ml) and the remaining two subjects had serum NGF concentrations ranging from 15 pg/ml to 116 pg/ml.
Overall, there was no apparent relationship between OXERVATE treatment and serum NGF concentrations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Animal studies have not been conducted to determine the carcinogenic and mutagenic potential of cenegermin-bkbj.Impairment of fertility
Daily subcutaneous administration of cenegermin-bkbj to male and female rats for at least 14 days prior mating, and at least 18 days post-coitum had no effect on fertility parameters in male or female rats at doses up to 267 mcg/kg/day (1709 times the MRHOD).In general toxicology studies, subcutaneous and ocular administration of cenegermin-bkbj in females was associated with ovarian findings including persistent estrus, ovarian follicular cysts, atrophy/reduction of corpora lutea, and changes in ovarian weight at doses greater than or equal to 19 mcg/kg/day (119 times the MRHOD).
-
14 CLINICAL STUDIES
The efficacy and safety of OXERVATE for the treatment of neurotropic keratitis was studied in a total of 151 patients, evaluated in two 8-week, randomized, multi-center, double-masked, vehicle-controlled studies. Patients were randomized to OXERVATE, cenegermin-bkbj 10 mcg/mL, or vehicle in Study NGF0212, and OXERVATE or vehicle in Study NGF0214 dosed 6 times daily in the affected eye(s) for 8 weeks. In study NGF0212, only patients with unilateral disease were enrolled, while in study NGF0214 patients with bilateral disease were treated bilaterally. The mean age was 61 to 65 years (18-95). The majority of patients were female (approximately 61%).
Table 1 below summarizes the results for complete corneal healing defined as absence of staining of the corneal lesion and no persistent staining in the rest of the cornea after 8 weeks of treatment.
Table 1. Percentage of Patients with Complete Corneal Healing at Week 8
Patients without any post-baseline measurements were excluded from the analysis. * p-value < 0.01 for both studies. Study
OXERVATE
Vehicle
Treatment Difference*
(95% CI)NGF0214
15/23
(65.2%)4/24
(16.7%)48.6%
(24%, 73.1%)NGF0212
36/50
(72.0%)17/51
(33.3%)38.7%
(20.7%, 56.6%)In patients who were healed after 8 weeks of treatment with OXERVATE, recurrences occurred in approximately 20% of patients in Study NGF0212 and 14% of patients in Study NGF0214.
The results of the mean change from baseline in corneal sensitivity inside the lesion after 8 weeks of treatment are summarized descriptively in Table 2. The mean changes in corneal sensitivity were not clinically significant in either study.
Table 2: Mean Corneal Sensitivity inside the Lesion: Baseline and Change from Baseline at Week 8
Change from baseline in corneal sensitivity inside the lesion was analyzed using an analysis of covariance model adjusting for baseline values. Patients without any post-baseline measurements were excluded from the analysis. *Mean (standard deviation) are presented at baseline; least squared means (standard error) are presented at Week 8 ** NGF0214: OXERVATE, n = 21; Vehicle, n = 23 NGF0212: OXERVATE, n = 48; Vehicle, n = 47 Study
Visit*
OXERVATE
Vehicle
Treatment Difference**
(95% CI)NGF0214
Baseline
0.8 (1.19)
0.6 (0.70)
Change from baseline at Week 8
1.6 (0.26)
0.7 (0.25)
0.9 (0.2, 1.7)
NGF0212
Baseline
1.1 (1.34)
1.0 (1.19)
Change from baseline at Week 8
1.1 (0.23)
0.8 (0.23)
0.3 (-0.4, 0.9)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OXERVATE (cenegermin-bkbj) ophthalmic solution, 0.002% (20 mcg/mL), is a sterile, preservative free clear, colorless solution in a multiple-dose vial, closed with a rubber stopper (not made with natural rubber latex), and an aluminum overseal with a polypropylene flip-off cap.
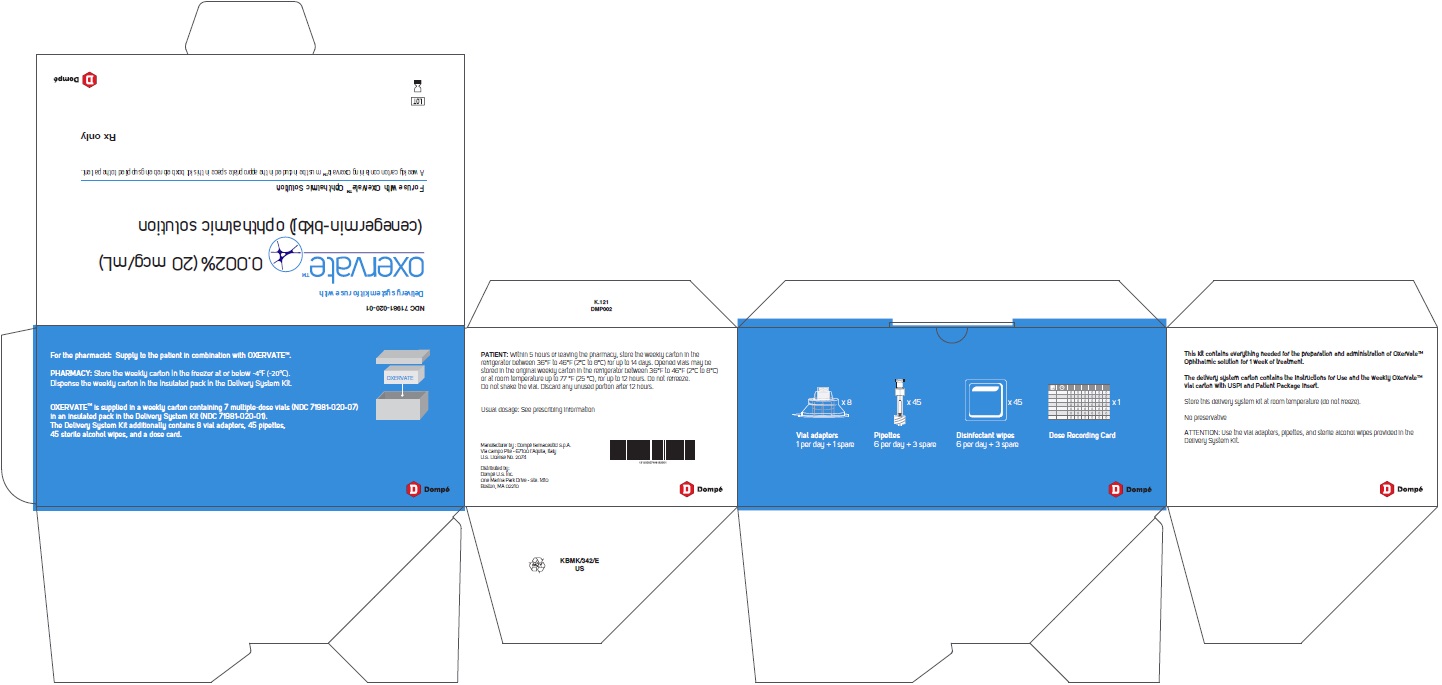
OXERVATE is supplied in weekly cartons containing 7 multiple-dose vials (NDC: 71981-020-07) in an insulated pack with the Delivery System Kit (NDC: 71981-001-01). The Delivery System Kit contains 8 vial adapters, 45 pipettes, 45 sterile disinfectant wipes, and 1 Dose Recording Card.
Pharmacy Storage
Store the weekly cartons in the freezer at or below -4°F (-20°C). Supply the weekly cartons in an insulated pack in combination with the Delivery System Kit.Patient Storage
Within 5 hours of delivery, store the weekly carton(s) containing OXERVATE vials in the refrigerator between 36°F to 46°F (2°C to 8°C) for up to 14 days. A vial opened for daily use may be stored in the original weekly carton in the refrigerator between 36°F to 46°F (2°C to 8°C) or at room temperature up to 77°F (25°C), for up to 12 hours [see Dosage and Administration (2.1)]. Do not refreeze the vials. Do not shake the vials. Discard the opened vial after 12 hours even if there is still some solution left inside. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Handling the Vials and the Delivery System
Advise patients that OXERVATE should be administered using the vial adapters, pipettes, and sterile disinfectant wipes provided in the Delivery System Kit and according to the instructions [see Dosage and Administration (2)]. One individual pipette should be used per application.Use with Contact Lenses
Advise patients that contact lenses should be removed before applying OXERVATE and to wait 15 minutes after instillation of the dose before reinserting the contact lenses into the eyes [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].Use with other topical products
Advise the patient to administer the eye drops at least 15 minutes apart, if more than one topical ophthalmic product is being used to avoid diluting products. Administer OXERVATE 15 minutes prior to using any eye ointment, gel or other viscous eye drops.Delayed or Missed Dose
If a dose is missed, treatment should be continued as normal, at the next scheduled administration.Storage Information
Instruct the patient to remove the weekly carton(s) containing 7 OXERVATE vials from the insulated pack within 5 hours of receiving it from the pharmacy and store the weekly carton(s) in the refrigerator [36°F to 46°F (2°C to 8°C)].Instruct the patient to only remove the number of OXERVATE vials from the weekly carton required for use over the course of a single day. Do not shake the vial.
Once opened, the vial can be kept in the original weekly carton in the refrigerator between 36°F to 46°F (2°C to 8°C) for up to 12 hours or at room temperature up to 77°F (25°C), but must be used within 12 hours. After 12 hours, advise patients to discard the vial with any unused amount.
Manufactured by
Dompé farmaceutici S.p.A.
Via Campo di Pile
67100 L’Aquila, Italy
U.S. License No. 2074™ Dompé 2017.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
OXERVATE (ox'-er-vayt)
(cenegermin-bkbj)
ophthalmic solution,
for topical ophthalmic use
What is OXERVATE?
OXERVATE is a prescription eye drop solution used to treat a condition called neurotrophic keratitis.
OXERVATE is safe and effective in children two years of age and older.
Before you use OXERVATE, tell your doctor about all of your medical conditions, including if you:
- have an infection in your eye. If you get an eye infection while using OXERVATE, talk your doctor right away.
- are using any other eye drops.
- wear contact lenses.
- are pregnant or plan to become pregnant. It is not known if OXERVATE will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if OXERVATE passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use OXERVATE.
Tell your doctor about all the medicines you take, including prescription and over-the counter medicines, vitamins, and herbal supplements.
How should I use OXERVATE?
- See the complete Instructions for Use at the end of this Patient Information leaflet for detailed instructions about the right way to use OXERVATE.
- Use OXERVATE exactly as your doctor tells you.
- Use 1 drop of OXERVATE in the affected eye or both eyes if needed, 6 times each day, about 2 hours apart starting in the morning. Continue your treatment for 8 weeks.
- If you use any other eye drops, wait at least 15 minutes before or after using OXERVATE. This will help to avoid one eye drop diluting the other eye drop.
- If you also use an eye ointment or gel or an eye drop that is thick, use OXERVATE first, and then wait at least 15 minutes before using the other eye ointment, gel, or drops.
- If you wear contact lenses in your affected eye or both eyes remove them before using OXERVATE and wait 15 minutes after using OXERVATE before reinserting them.
- If you miss a dose of OXERVATE, take your next dose at your scheduled time. Do not take an extra dose to make up for a missed dose.
- Do not use other eye medicines without talking to your doctor.
- Talk to your doctor first before you stop using OXERVATE.
- If you have any questions about how to use OXERVATE, ask your doctor or pharmacist.
What should I avoid while using OXERVATE?
Your vision may be blurred for a short time after using OXERVATE. If this happens, wait until your vision clears before you drive or use machines.
What are the possible side effects of OXERVATE?
The most common side effect of OXERVATE is eye pain, enlarged blood vessels in the white of the eyes (ocular hyperemia), swelling (inflammation) of the eye, and increase of tears (increased lacrimation).
Tell your doctor if you have any side effects that bother you. These are not all the possible side effects of OXERVATE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of OXERVATE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OXERVATE for a condition for which it was not prescribed. Do not give OXERVATE to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about OXERVATE that is written for health professional.
What are the ingredients in OXERVATE?
Active ingredient: cenegermin-bkbj
Inactive ingredients: disodium hydrogen phosphate anhydrous, hydroxypropylmethyl cellulose, L‑methionine, mannitol, polyethylene glycol 6000, sodium dihydrogen phosphate dihydrate, trehalose dihydrate, Water for Injection, USP, and hydrochloric acid and/or sodium hydroxide to adjust pH.
Manufactured by:
Dompé farmaceutici S.p.A.
Via Campo di Pile
67100 L’Aquila, Italy
U.S. License No. 2074
Manufactured for: Dompé U.S. Inc.
One Marina Park Drive - Ste. 1410, Boston, MA 02210
For more information, go to www.oxervate.com or call 1-833-366-7387.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised or Issued: October 2019
-
Instructions for Use
OXERVATETM (ox'-er-vayt)
(cenegermin-bkbj)
ophthalmic solution, for topical ophthalmic useRead this Instructions for Use before you start using OXERVATETM and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition, or your treatment. Only you and your doctor can decide if OXERVATE is right for you. Share the important information in this leaflet with members of your household.
Important:
- OXERVATE is for use in the eye.
- Do not shake the OXERVATE vial.
- Use OXERVATE with the vial adapters, sterile disinfectant wipes, and pipettes that come with your Delivery System Kit.
OXERVATE is supplied in an insulated pack, in weekly cartons containing 7 multiple-dose vials. OXERVATE is supplied with an accompanying Delivery System Kit. The Delivery System Kit will have medical devices for withdrawing and using OXERVATE.
You will receive both the OXERVATE weekly carton(s) and the Delivery System Kit from the pharmacy.
The OXERVATE carton contains:
- 7 multiple-dose vials of OXERVATE (1 vial per day of the week)
The Delivery System Kit contains the following:
- 7 vial adapters
- 42 pipettes
- 42 sterile disinfectant wipes and
- Dose Recording Card (1)
- Extra adapter (1) pipettes (3) and wipes (3) are included as spares.
How should I store OXERVATE?
- Remove the weekly carton(s) of OXERVATE from the insulated pack and store it for up to 14 days in a refrigerator in the original carton as soon as you can.
- Store the weekly carton(s) at 36°F to 46°F (2°C to 8°C) no later than 5 hours from when you receive the medicine from your pharmacy. Do not freeze.
- OXERVATE is stored in a freezer at the pharmacy. If you start treatment right away after receiving the weekly carton, you will have to wait until the first vial is thawed. Thaw the vial at room temperature up to 77°F (25°C). Thawing the vial could take up to 30 minutes when kept at room temperature.
- Keep all medicines out of the reach of children.
Follow Steps 1 to 19 each day you use OXERVATE.
Gather your supplies:
- Remove 1 vial of OXERVATE from the refrigerator in the morning (always at the same time each morning) to use during the day. If OXERVATE is to be used in both eyes, remove 2 vials from the refrigerator.
- 1 vial adapter
- 1 pipette (if both eyes 2 pipettes)
- 1 sterile disinfectant wipe (if both eyes 2 sterile disinfectant wipes)
- Dose Recording Card
Step 1. Wash your hands.
Step 2. If you wear contact lenses, take them out before using OXERVATE.
Step 3. Remove the plastic flip-off cap from the vial.

Step 4. Peel-off the back of the vial adapter blister pack.

Step 5. Without removing the vial adapter from its blister pack, connect it to the vial by firmly pushing it down until it snaps into place over the neck of the vial. The spike of the vial adapter should pierce through the vial’s rubber stopper. After the vial adapter has been connected correctly, do not remove it from the vial.
Note: After the vial adapter is connected to the vial, OXERVATE can be stored in the refrigerator between 36°F to 46°F (2°C to 8°C) for up to 12 hours.
If needed, the OXERVATE with the connected vial adapter may be stored at room temperature up to 77°F (25°C).
Step 6. Remove and throw away the packaging of the vial adapter.
The multiple-dose vial of OXERVATE is now ready for use (1 drop in the affected eye every 2 hours 6 times a day).
To withdraw and give each dose of OXERVATE, follow Steps 7 to 19:
Step 7. Take a single sterile disinfectant wipe and gently clean the surface of the valve on the connector part of the vial adapter.
After cleaning, wait for about 1 minute to allow the valve to dry.
Step 8. Remove a pipette from its protective packaging.

Step 9. Screw the pipette (clockwise) into the connector part of the vial adapter.
Step 10. Make sure that the pipette plunger is pushed all the way down.

Step 11. Turn the vial upside-down with the pipette still connected. Gently pull the plunger until it stops, to draw the eye drop solution into the pipette. Make sure the plunger has reached the stop point.
Step 12. Check the pipette to make sure it contains the eye drop solution. Air bubbles may cause blockage and prevent the pipette from filling properly (especially the first time you withdraw the eye drop solution). If the pipette is empty, keep the vial with the connected pipette upside-down, push the plunger all the way in and pull it out again.

Step 13. After the pipette has been correctly filled, unscrew the pipette from the connector part of the vial adapter (counter-clockwise). Pull the pipette straight up to remove it.

Step 14.
- Sit or lie down to steady yourself when you instill OXERVATE.
- Holding the pipette, pointing down, between your middle finger and thumb, tilt your head back and position the pipette above your affected eye.
- With your other hand, pull down your lower eyelid, increasing the space between the inner eyelid and the eyeball (the conjunctival fornix).
- Gently push the plunger down until at least a drop is released into the conjunctival fornix.
- Make sure you do not touch your eye with the tip of the pipette.
- With your head still tilted back, blink a few times so that the medicine covers the surface of your eye.

Step 15. Throw away the used pipette right away after use, even if there is still some eye drop solution left in it.
Only use 1 pipette for each eye and each dose.
If you miss your eye and there is no longer any eye drop solution in the pipette, try again, using a new pipette and wipe (See Steps 7 to 14).
Step 16. After each use throughout the day, place the vial back in the refrigerator or keep it below 77°F (25°C) for the rest of the day, with the vial adapter still connected.

Step 17. Repeat from Step 7 to Step 16 every 2 hours 6 times a day, using a new sterile disinfectant wipe and a new pipette each time.
If you use drops in both eyes, repeat the above instructions for your other eye using a new pipette. You will need to use 2 vials each day.
Store the vial at or below 77ºF (25ºC) throughout the day. You can also store the vial in the refrigerator but do not freeze the vial.
Step 18. Throw away the used vial at the end of each day even if there is still some eye drop solution left in it. Throw away the vial no later than 12 hours from the time you connected the vial adapter to it even if there is eye solution still left in the vial.

Step 19. Track each time you instill an eye drop of OXERVATE on the weekly Dose Recording Card provided with the delivery system.
This will allow you to track your 6 doses each treatment day, the date of the first use of the weekly supply and the time of the vial opening which is when you connect the vial adapter to the vial during the week.

To make sure accurate dosing every 2 hours, you may want to set an alarm as a reminder for dosing.
Manufactured by:
Dompé farmaceutici S.p.A.
Via Campo di Pile
67100 L’Aquila, Italy
U.S. License No. 2074Manufactured for: Dompé U.S. Inc.
One Marina Park Drive - Ste. 1410, Boston, MA 02210™ Dompé 2017.
This Instructions for Use has been approved by the U.S Food and Drug Administration
Issued: October 2019.
-
PRINCIPAL DISPLAY PANEL - Carton
NDC: 71981-020-07
OxervateTM 0.002% (20 mcg/mL)
(cenegermin-bkbj) ophthalmic solution7 X 1 mL multiple-dose vial
Sterile
For topical application in the eyeRx only

-
PRINCIPAL DISPLAY PANEL- Delivery System Kit
NDC: 71981-001-01
Delivery system kit for use with
OxervateTM(cenegermin-bkbj ophthalmic
solution) 0.002% (20 mcg/mL)For use with OxervateTM Ophthalmic Solution
A weekly carton containing OxervateTM must be included in the appropriate space in this kit box before being supplied to the patient.
Rx only
Dompé
-
INGREDIENTS AND APPEARANCE
OXERVATE
cenegermin-bkbj kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71981-001 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71981-001-01 1 in 1 KIT 11/26/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 1 mL Part 1 of 1 OXERVATE
cenegermin-bkbj solution/ dropsProduct Information Item Code (Source) NDC: 71981-020 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CENEGERMIN (UNII: B6E7K36KT8) (CENEGERMIN - UNII:B6E7K36KT8) CENEGERMIN 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 47.03 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 12.22 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) 2.87 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 1.22 mg in 1 mL HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) 1.0 mg in 1 mL POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) 10.0 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.01 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) NITROGEN (UNII: N762921K75) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 7 in 1 CARTON 1 1 mL in 1 VIAL, MULTI-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761094 11/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761094 11/26/2018 OXERVATE
cenegermin-bkbj solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71981-020 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CENEGERMIN (UNII: B6E7K36KT8) (CENEGERMIN - UNII:B6E7K36KT8) CENEGERMIN 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 47.03 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 12.22 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) 2.87 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) 1.22 mg in 1 mL HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) 1.0 mg in 1 mL POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) 10.0 mg in 1 mL METHIONINE (UNII: AE28F7PNPL) 0.01 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) NITROGEN (UNII: N762921K75) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71981-020-07 7 in 1 CARTON 11/26/2018 1 1 mL in 1 VIAL, MULTI-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761094 11/26/2018 Labeler - Dompé farmaceutici S.p.A. (428166094)
Trademark Results [OXERVATE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OXERVATE 79205837 5352715 Live/Registered |
DOMPE' FARMACEUTICI S.P.A. 2016-12-27 |
 OXERVATE 79205724 5357221 Live/Registered |
DOMPE' FARMACEUTICI S.P.A. 2016-12-27 |
 OXERVATE 79170436 4868952 Live/Registered |
Dompé farmaceutici S.p.A. 2015-05-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.