Ibuprofen by Medline Industries / P & L Development, LLC DRUG FACTS
Ibuprofen by
Drug Labeling and Warnings
Ibuprofen by is a Otc medication manufactured, distributed, or labeled by Medline Industries, P & L Development, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
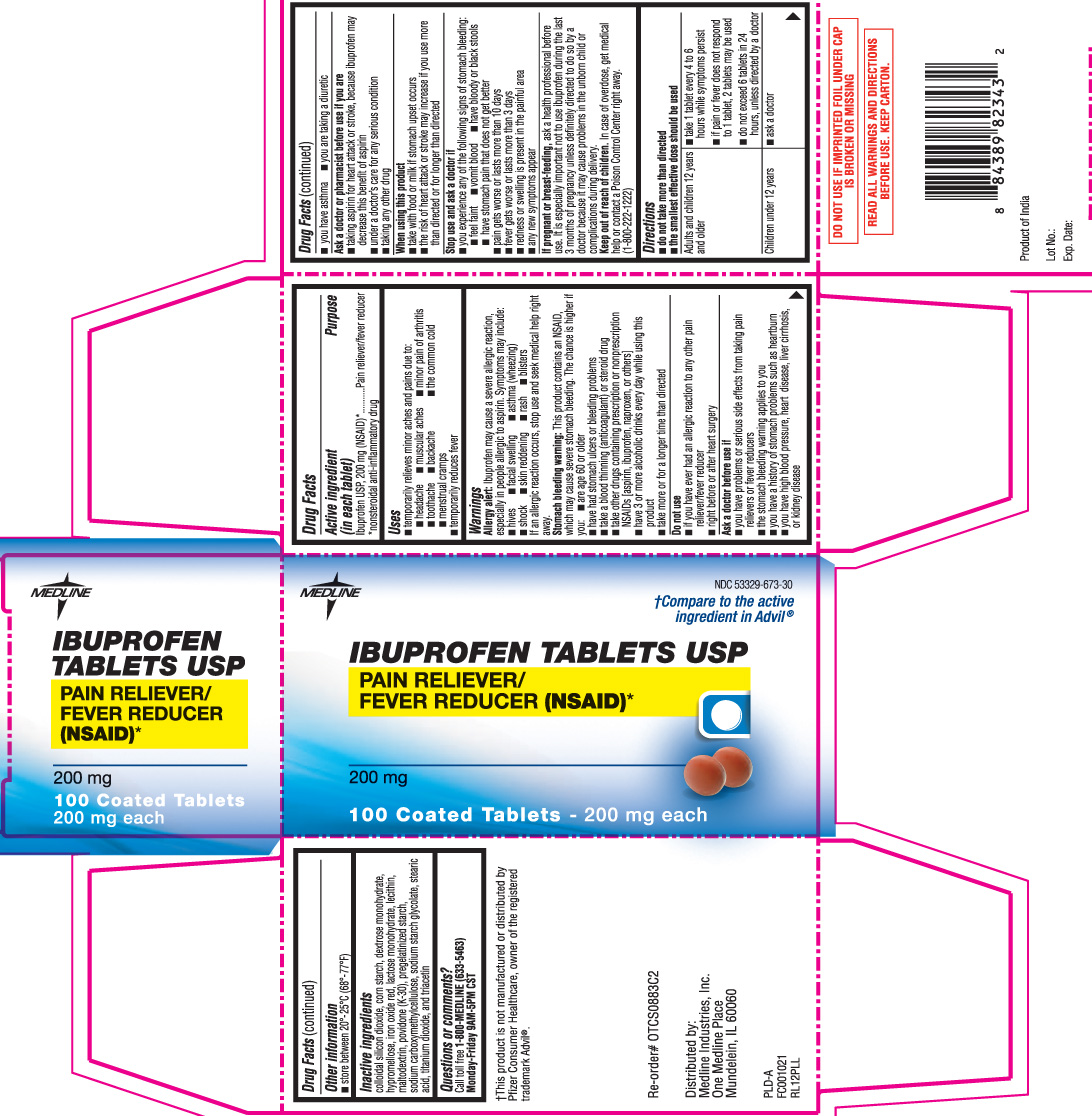
IBUPROFEN- ibuprofen tablet
Medline Industries
----------
DRUG FACTS
Active Ingredient (in each tablet)
Ibuprofen USP, 200 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Uses
- temporarily relieves minor aches and pains due to:
- Headache
- muscular aches
- minor pain of arthritis
- toothache
- backache
- the common cold
- menstrual cramps
- temporarily reduces fever
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use an seek medical help right away.
Stomach bleeding warning: This product contains an NSAID which may cause severe stomach bleeding. The chance are higher if you:
- are age 60 or oder
- have had stomach ulders or bleeding problems
- take blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescritpion or non-prescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks everyday while using this prdouct
- take more or for a longer time than directed.
Do not use
- if you ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- you have problems or serious side effects from taking pain relievers or fever reducers
- the stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic.
Ask a doctor or pharmacist before use if you are
- taking aspirin to prevent heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- under a doctor's care for any serious condition
- taking any other drug.
When using this product
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed.
Stop use and ask a doctor if
- You experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not bet better
- Pain gets worse or last more than 10 days
- Fever gets worse or last more than 3 days
- Redness or swelling is present in the painful area
- Any new symptoms appear
If pregnant or breast-feeding,
ask a health professional before use. It is especially important not to use ibuprofen druing the last 3 months of pegnancy unless defintely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222)
Directions
- do not take more than directed
- the smallest effective dose should be used
| Adults and children 12 years and older |
|
| children under 12 years |
|
Inactive Ingredients
colloidal silicon dioxide, corn starch, dextrose monohydrate, hypromellose, iron oxide red, lactose monohydrate, lecithin, maltodextrin, povidone (K-30), pregelatinized starch, sodium carboxymethylcellulose, sodium starch glycolate, stearic acid, titanium dioxide, and triacetin
Principal Display Panel
†Compare to the active ingredient in ADVIL®
Ibuprofen Tablets USP
Pain Reliever/ Fever Reducer (NSAID)*
†This product is not manufactured or distributed by Pfizer Consumer Healthcare, owner of the registered trademark Advil®
DO NOT USE IF IMPRINTED FOIL UNDER CAP IS BROKEN OR MISSING
READ ALL WARNINGS AND DIRECTIONS BEFORE USE. KEEP CARTON.
PRODUCT OF INDIA
Distributed by:
Medline Industries, Inc.
One Medline Place
Mundelein, IL 60060
| IBUPROFEN
ibuprofen tablet |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Medline Industries (025460908) |
| Registrant - P & L Development, LLC (079765031) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.