VIRASAL- salicylic acid solution
Virasal by
Drug Labeling and Warnings
Virasal by is a Prescription medication manufactured, distributed, or labeled by Elorac, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle - Package Insert Text:
DESCRIPTION
Virasal is a topical preparation containing 27.5% salicylic acid in a proprietary film-forming vehicle that comprises isopropyl alcohol, butyl acetate, polyvinyl butyral, isopropyl metacresol, trimethyl pentanyl diisobutyrate, phenic acid and acrylates copolymer. The pharmacologic activity of Virasal is generally attributed to the keratolytic activity of salicylic acid which is incorporated into a polyacrylic, film-forming virucidal vehicle designed to cover the wart without the need for a bandage. The structural formula of salicylic acid is:
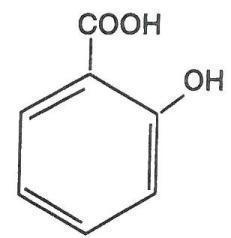
CLINICAL PHARMACOLOGY
Although the exact mode of action for salicylic acid in the treatment of warts is unknown, its activity appears to be associated with its keratolytic action, which results in mechanical removal of epidermal cells infected with wart viruses.
The virucidal complex incorporated into Virasal’s proprietary vehicle is designed to help reduce risk of reinfection at the wart site, as well as prevent viral contamination of the product under normal usage.
INDICATIONS AND USAGE
Virasal is indicated for the topical treatment and removal of common warts and plantar warts.
CONTRAINDICATIONS
Patients with diabetes or impaired blood circulation should not use Virasal. Virasal also should not be used on moles, birthmarks, and unusual warts with hair growing from them, or warts on the face.
PRECAUTIONS
Virasal is for external use only. Do not permit Virasal to contact eyes or mucous membranes. If contact with eyes or mucous membranes occurs, immediately flush with water for 15 minutes. Virasal should not be allowed to contact normal skin surrounding wart, since localized irritation may occur. Treatment should be discontinued if excessive irritation occurs. Virasal is flammable. Keep away from fire or flame. Keep bottle tightly capped when not in use.
ADVERSE REACTIONS
A localized irritant reaction may occur if Virasal is applied to the normal skin surrounding the wart. Any irritation may normally be controlled by temporarily discontinuing use and by applying the medication only to the wart site when treatment is resumed.
DOSAGE AND ADMINISTRATION
Prior to applying Virasal, soak wart in warm water for five minutes. Remove any loosened tissue by gently rubbing with a brush, wash cloth, or emery board. Dry wart site thoroughly. Using the brush applicator supplied, apply Virasal twice to entire wart surface, allowing the first application to dry before applying the second. Continue treatment once or twice a day as directed by physician. Be careful not to apply to surrounding skin.
Clinically visible improvement will normally occur during the first or second week of therapy. Maximum resolution may be expected after four to six weeks of Virasal use.
HOW SUPPLIED
Virasal is supplied in 10ml amber bottles with a brush applicator (NDC: 42783-312-10).
Store at controlled room temperature, 15° to 30°C (59° to 86°F).
Manufactured for:
Elorac, Inc.
Vernon Hills, IL 60061U.S. Patent Pending
Revised 06/2011
221619
-
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle - Container Label Principal Display Panel Text:
NDC: 42783-312-10
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle
Wart Remover
FOR TOPICAL USE ONLY
NOT FOR USE IN THE EYESRx only
1/3 fl. oz (10 mL)

-
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle - Carton Label Principal Display Panel Text:
NDC: 42783-312-10
Virasal (27.5% Salycylic Acid) Antiviral Film-Forming Vehicle
Wart Remover
FOR DERMATOLOGICAL USE ONLY
NOT FOR USE IN THE EYESRx only
1/3 fl. oz (10 mL)
-
INGREDIENTS AND APPEARANCE
VIRASAL
salicylic acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42783-312 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (salicylic acid - UNII:O414PZ4LPZ) salicylic acid 275 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42783-312-10 1 in 1 CARTON 04/01/2011 1 10 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/01/2011 Labeler - Elorac, Inc. (832590009) Registrant - Elorac, Inc. (832590009)
Trademark Results [Virasal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIRASAL 85177916 4003973 Live/Registered |
Elorac, Inc 2010-11-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.