4357 First Aid Kit by Honeywell Safety Products USA, INC 4357 FIRST AID KIT kit
4357 First Aid Kit by
Drug Labeling and Warnings
4357 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Eyewash Active ingredient
- Eyewash Purpose
- Eyewash Uses
-
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyewash Directions
- Eyewash Inactive ingredients
- Eyewash Questions
- Burn Relief Water Soluble Active ingredients
- Burn Relief Water Soluble Purpose
- Burn Relief Water Soluble Uses
-
Burn Relief Water Soluble
Warnings
For external use only
Flammable keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperatures above 120 0 F
Do not use
- in or near the eyes or other mucous membranes
- in case of serious burns
- in case of deep or puncture wounds
- for prolonged period of time
- on large portion of the body
- Burn Relief Water Soluble Directions
- Burn Relief Water Soluble Other information
- Burn Relief Water Soluble Inactive ingredients
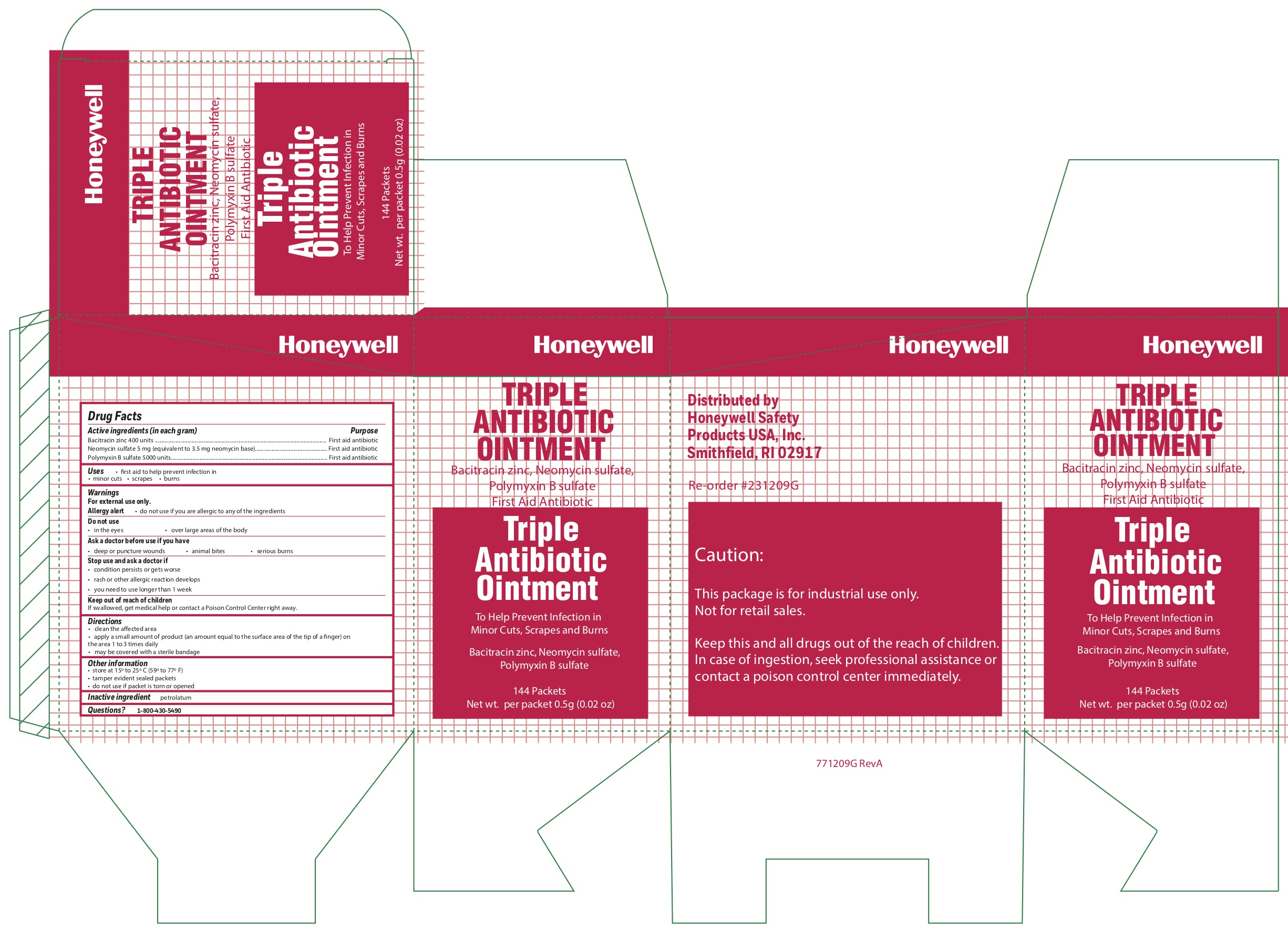
- Triple Active ingredients
- Triple Purpose
- Triple Uses
- Triple Warnings
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
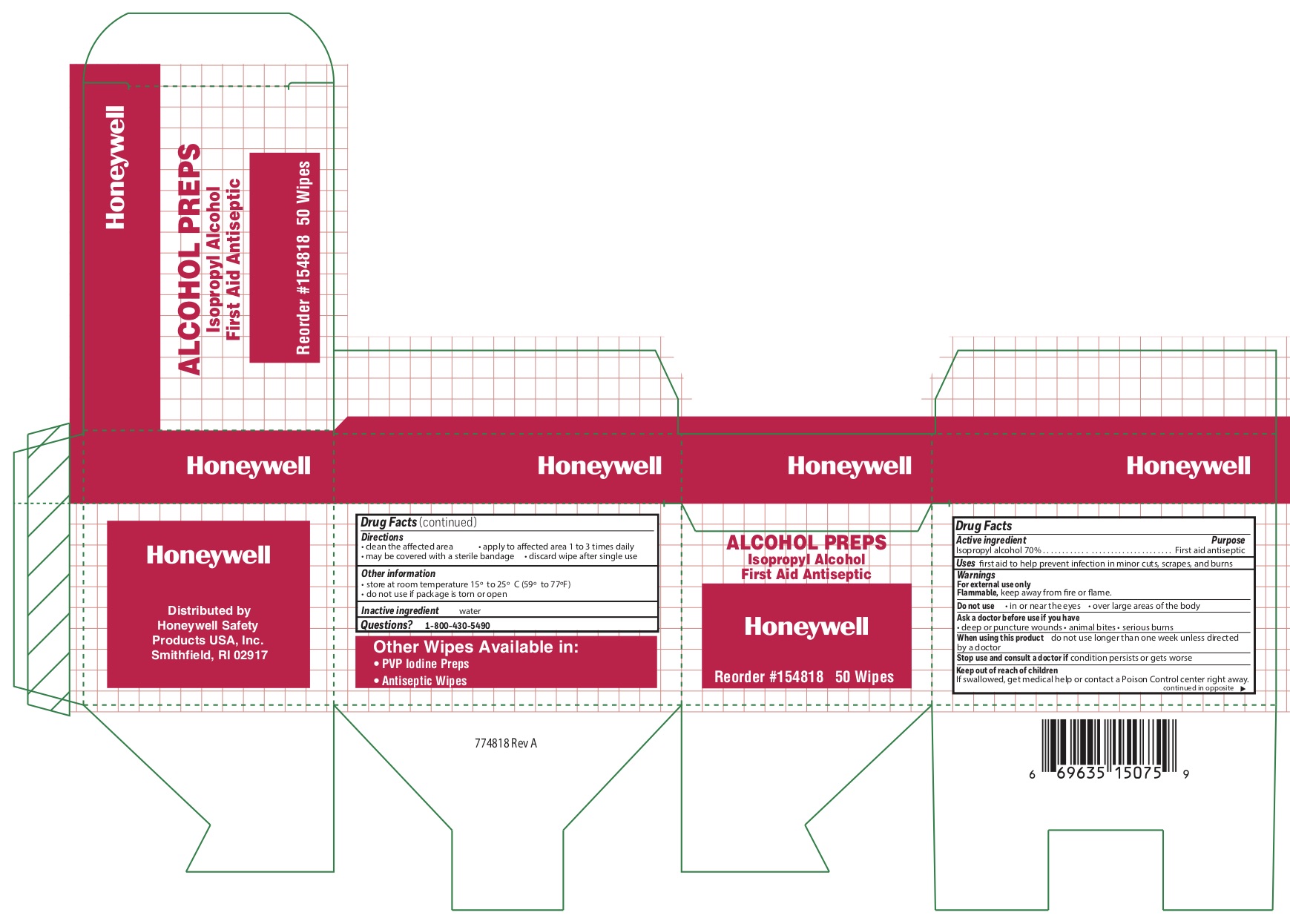
- Alcohol Active ingredient
- Alcohol Purpose
- Alcohol Uses
- Alcohol Warnings
- Alcohol Directions
- Alcohol Other information
- Alcohol Inactive ingredient
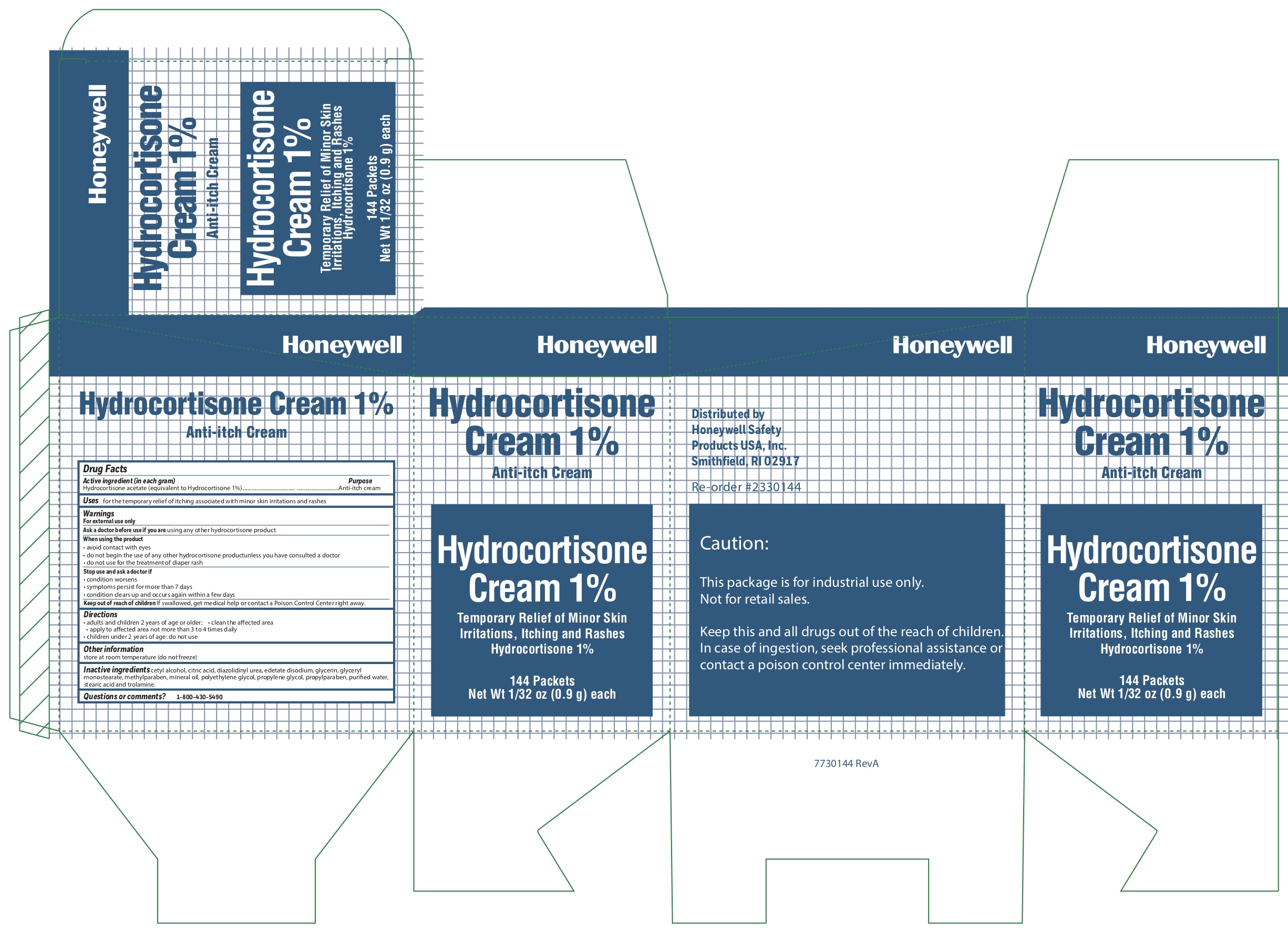
- Hydrocortisone Active ingredient (in each gram)
- Hyrdocortisone Purpose
- Hydrocortisone Uses
-
Hydrocortisone
Warnings
For external use only
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
- Hydrocortisone Directions
- Hydrocortisone Other information
- Hydrocortisone Inactive ingredients
- Hydrocortisone Questions or Comments?
- Foille Active ingredient
- Foille Purpose
- Foille Uses
- Foille Warnings
- Foille Directions
- Foille Other information
- Foille Inactive ingredients
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn Jel Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
-
4357
6832649 KIT CONTENTS
1 BLUE DETEC FNGERTP 8 WVN 25/B
1 BLUE DETEC KNUCKLE WVN 40/BX
1 BLUE DETEC 1X3 WVN 100/BX
1 INSTANT COLD PACK 4" X 6"
5 BURN JEL 1/8 OZ, 6 PER
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 FIRST AID GUIDE ASHI
1 GAUZE CLEAN-WRAP BDGE N/S 2"
1 GZE PADS STERILE 2"X 2" 25'S
1 GZE PADS STERILE 3"X 3" 25'S
1 CO-FLEX BANDAGE 2"X 5YDS TAN
1 ALCOHOL WIPES 50'S
1 TRIPLE BIOTIC .5 GRAM PKT 20
1 HYDROCORTISONE 1% .9 GRM 20'S
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 SPLINTER OUT 10 PIECES/PK
1 KIT TWEEZER 3 1/2" SLANTED
1 F A KIT EMPTY BLANK 140
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
5 WATER-JEL BURN DRESSING 4 X 4
3 FOILLE BURN .5OZ 2'S
- Eyewash Principal Display Panel
- Burn Relief Water Soluble Principal Display Panel
- Triple Principal Display Panel
- Alcohol Principal Display Panel
- Hydrocortisone Principal Display Panel
- Foille Principal Display Panel
- Burn Jel Principal Display Panel
- 4357 Kit Label 6832649
-
INGREDIENTS AND APPEARANCE
4357 FIRST AID KIT
4357 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4357 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4357-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 20 PACKET 18 g Part 2 1 BOTTLE 118 mL Part 3 1 CAN 85 g Part 4 20 PACKET 10 g Part 5 50 POUCH 20 mL Part 6 20 PACKET 18 g Part 7 6 TUBE 84 g Part 8 30 PACKET 105 g Part 1 of 8 HYDROCORTISONE
anti-itch creamProduct Information Item Code (Source) NDC: 0498-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0801-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/15/2019 Part 2 of 8 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC: 0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0100-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/18/2018 Part 3 of 8 BURN WATER SOLUBLE
benzocaine, benzethonium chloride, menthol sprayProduct Information Item Code (Source) NDC: 0498-0021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.2 g in 100 g BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 10 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.33 g in 100 g Inactive Ingredients Ingredient Name Strength ISOBUTANE (UNII: BXR49TP611) BUTANE (UNII: 6LV4FOR43R) PROPANE (UNII: T75W9911L6) DIPROPYLENE GLYCOL (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0021-40 85 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/12/2018 Part 4 of 8 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC: 0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0750-36 0.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 5 of 8 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 6 of 8 HYDROCORTISONE
anti-itch cream ointmentProduct Information Item Code (Source) NDC: 0498-0800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0800-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/06/2013 10/15/2019 Part 7 of 8 BLISTEX FOILLE MEDICATED FIRST AID
benzocaine and chloroxylenol ointmentProduct Information Item Code (Source) NDC: 10157-9302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5 g in 100 g CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM LAURYL SULFATE (UNII: 368GB5141J) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE CALCIUM DISODIUM ANHYDROUS (UNII: 8U5D034955) CERESIN (UNII: Q1LS2UJO3A) EUGENOL (UNII: 3T8H1794QW) MALEIC ANHYDRIDE (UNII: V5877ZJZ25) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) CORN OIL (UNII: 8470G57WFM) SODIUM BORATE (UNII: 91MBZ8H3QO) YELLOW WAX (UNII: 2ZA36H0S2V) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/05/2013 Part 8 of 8 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 manufacture(10157-9302) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4357) Establishment Name Address ID/FEI Business Operations Dixon Investments 115315822 manufacture(0498-0021) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0750, 0498-0800, 0498-0203, 0498-0801) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, Inc. 167518617 manufacture(0498-0100) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0143)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.