DRX CHOICE TENSION HEADACHE- acetaminophen, caffeine tablet, film coated
DRX CHOICE by
Drug Labeling and Warnings
DRX CHOICE by is a Otc medication manufactured, distributed, or labeled by Raritan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each caplet)
- Purposes
- Uses
-
Warnings
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Liver warning:
- This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Caffeine warning:
The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and occasionally, rapid heartbeat.
Do not use
- if you are allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
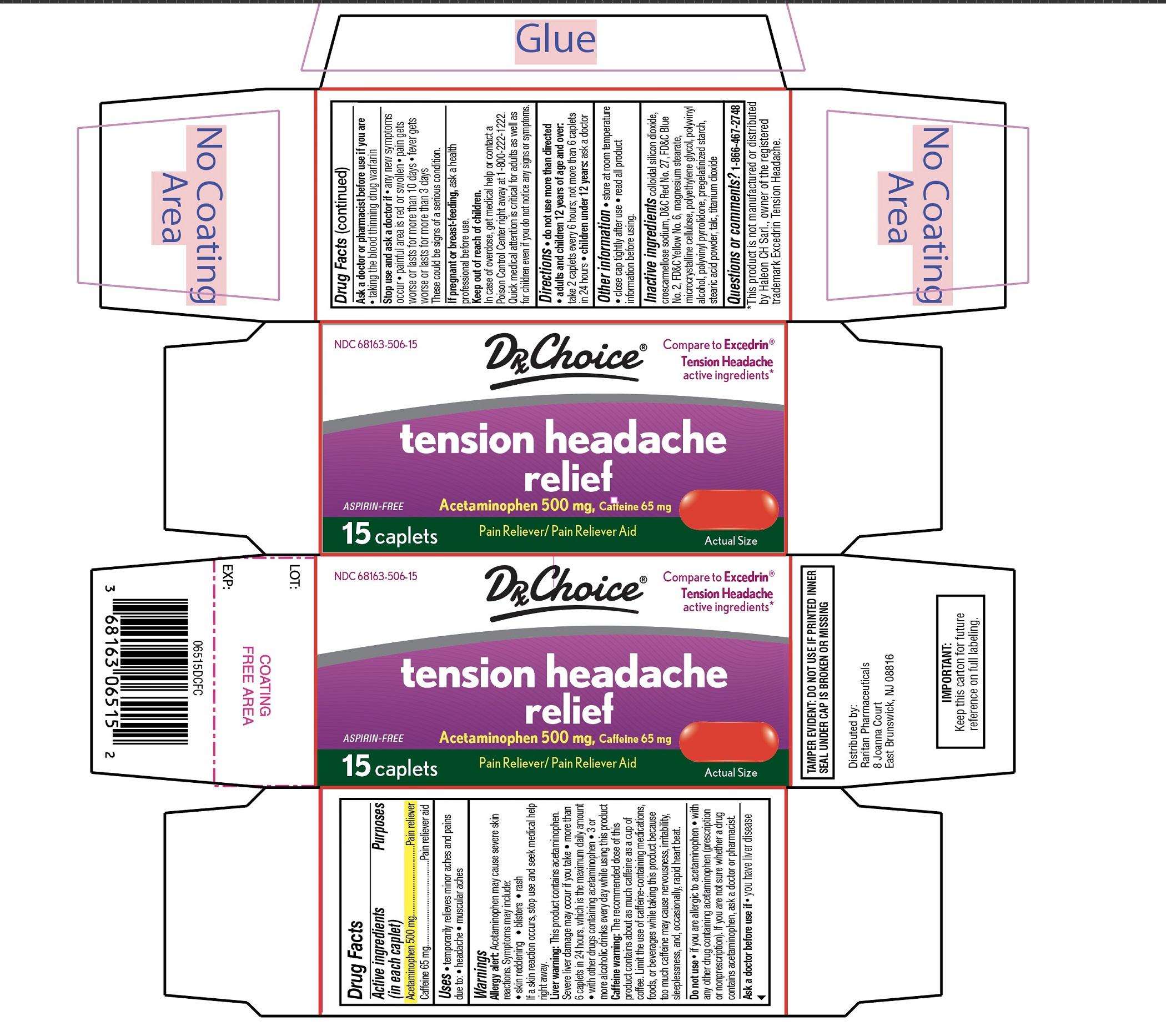
Principal Display Panel
Compare to Excedrin® Tension Headacheactive ingredients *
NDC: 68163-506-15
DRx Choice
tension headache
relief
ASPIRIN-FREE
Acetaminophen 500 mg,Caffeine 65 mg
Pain Reliever / Pain Reliever Aid
ACTUAL SIZE
15 CAPLETS
TAMPER-EVIDENT: DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING.
IMPORTANT:Keep this carton for future reference on full labeling.
Distributed by: Raritan Pharmaceuticals
8 Joanna Court
East Brunswick, NJ 08816
*This product is not manufactured or distributed by Haleon CH Sarl., owner of the registered trademark Excedrin Tension Headache.
-
INGREDIENTS AND APPEARANCE
DRX CHOICE TENSION HEADACHE
acetaminophen, caffeine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68163-506 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) EICOSYL POVIDONE (UNII: XQQ9MKE2BJ) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape OVAL (Caplet) Size 17mm Flavor Imprint Code RP065 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68163-506-15 1 in 1 CARTON 05/16/2025 1 15 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 05/16/2025 Labeler - Raritan Pharmaceuticals Inc. (127602287)
Trademark Results [DRX CHOICE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DRX CHOICE 88314307 5872019 Live/Registered |
Raritan Pharmaceuticals 2019-02-25 |
 DRX CHOICE 78431682 not registered Dead/Abandoned |
Raritan Pharmaceuticals Inc. 2004-06-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.