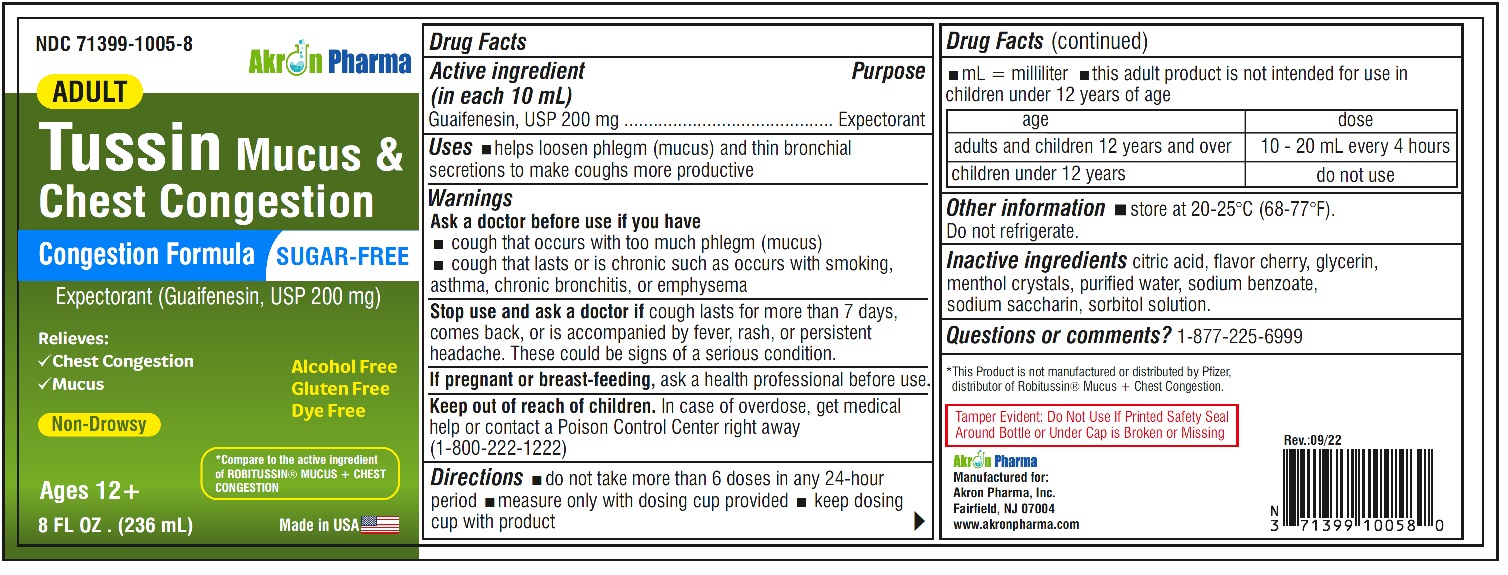
Adult Tussin Mucus and Chest Congestion DM Sugar Free by Akron Pharma Inc. / SLV PHARMACEUTICALS LLC Drug Facts
Adult Tussin Mucus and Chest Congestion DM Sugar Free by
Drug Labeling and Warnings
Adult Tussin Mucus and Chest Congestion DM Sugar Free by is a Otc medication manufactured, distributed, or labeled by Akron Pharma Inc., SLV PHARMACEUTICALS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ADULT TUSSIN MUCUS AND CHEST CONGESTION DM SUGAR FREE- dextromethorphan hbr, guaifenesin liquid
Akron Pharma Inc.
----------
Drug Facts
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- mL= milliliter
- this adult product is not intended for use in children under 12 years of age
- adults and children 12 years and over: 10- 20 mL every 4 hours
- children under 12 years: do not use
Other information
- store between 20-25ºC(68-77ºF). Do not refrigerate
- protect from light
- Pharmacist-Preserve and dispense in a tight, light-resistant container with a child resistant cap as defined in the USP.
| ADULT TUSSIN MUCUS AND CHEST CONGESTION DM SUGAR FREE
dextromethorphan hbr, guaifenesin liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Akron Pharma Inc. (067878881) |
| Registrant - SLV PHARMACEUTICALS LLC (081225162) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SLV PHARMACEUTICALS LLC | 081225162 | manufacture(71399-1005) | |
Revised: 1/2024
Document Id: 77a8f91c-a576-4b5d-abcb-bfee6cbf048d
Set id: 8a650a18-6738-4910-a663-731cc2cc93a1
Version: 2
Effective Time: 20240118
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.