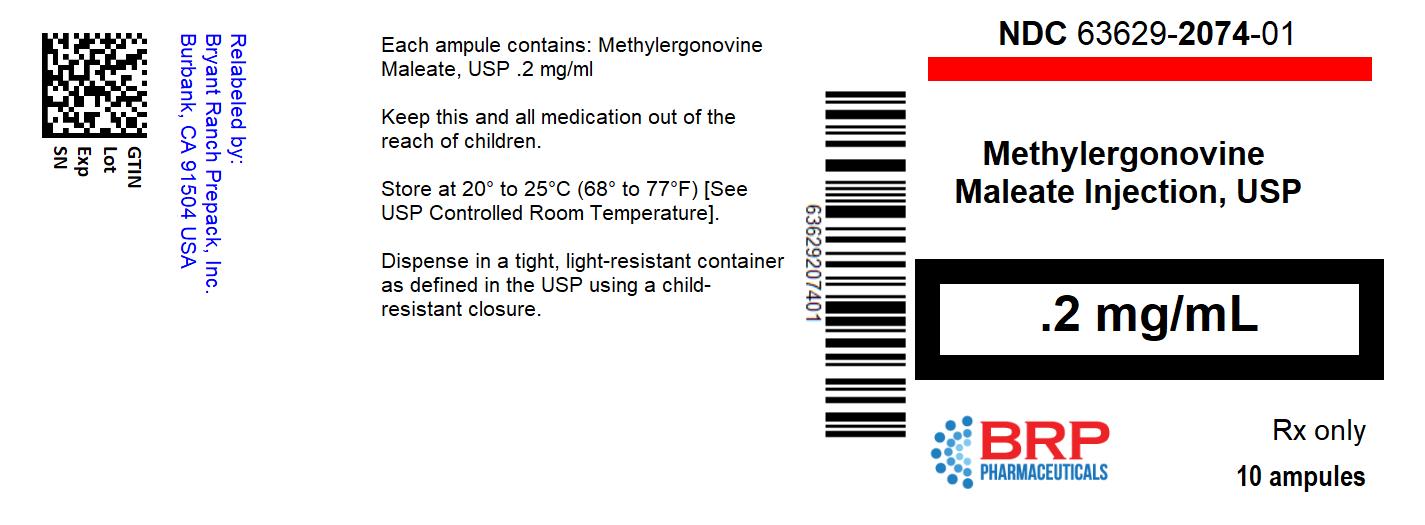
METHYLERGONOVINE MALEATE injection
METHYLERGONOVINE MALEATE by
Drug Labeling and Warnings
METHYLERGONOVINE MALEATE by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Methylergonovine Maleate Injection, USP is a semi-synthetic ergot alkaloid used for the prevention and control of postpartum hemorrhage.
Methylergonovine Maleate Injection, USP is available in sterile ampules of 1 mL, containing 0.2 mg methylergonovine maleate for intramuscular or intravenous injection.
Ampules, 1 mL, clear, colorless solution.
Active Ingredients: methylergonovine maleate, USP, 0.2 mg.
Inactive Ingredients: maleic acid, 0.10 mg; sodium chloride, 7.0 mg; water for injection, qs to 1 mL. Chemically, methylergonovine maleate is designated as ergoline-8-carboxamide, 9,10-didehydro-N-[l-(hydroxymethyl)propyl ]-6-methyl-, [8β(S)]-, (Z)-2-butenedioate (1:1) (salt). Its structural formula is:
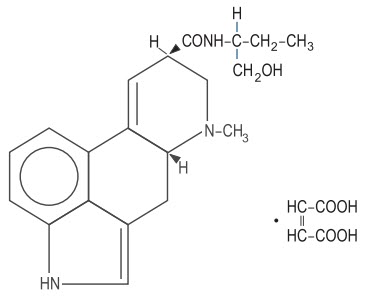
C20H25N3O2∙C4H4O4 Mol. wt. - 455.51
-
CLINICAL PHARMACOLOGY
Methylergonovine maleate acts directly on the smooth muscle of the uterus and increases the tone, rate, and amplitude of rhythmic contractions. Thus, it induces a rapid and sustained tetanic uterotonic effect which shortens the third stage of labor and reduces blood loss. The onset of action after I.V. administration is immediate; after I.M. administration, 2-5 minutes, and after oral administration, 5-10 minutes.
Pharmacokinetic studies following an I.V. injection have shown that methylergonovine is rapidly distributed from plasma to peripheral tissues within 2-3 minutes or less. The bioavailability after oral administration was reported to be about 60%, with no accumulation after repeated doses. During delivery with intramuscular injection, bioavailability increased to 78%. Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
Bioavailability studies conducted in fasting healthy female volunteers have shown that oral absorption of a 0.2 mg methylergonovine tablet was fairly rapid, with a mean peak plasma concentration of 3243 ± 1308 pg/mL observed at 1.12 ± 0.82 hours. For a 0.2 mg intramuscular injection, a mean peak plasma concentration of 5918 ± 1952 pg/mL was observed at 0.41 ± 0.21 hours. The extent of absorption of the tablet, based upon methylergonovine plasma concentrations, was found to be equivalent to that of the I.M. solution given orally, and the extent of oral absorption of the I.M. solution was proportional to the dose following administration of 0.1, 0.2, and 0.4 mg. When given intramuscularly, the extent of absorption of methylergonovine maleate solution was about 25% greater than the tablet. The volume of distribution (VdSS/F) of methylergonovine was calculated to be 56.1 ± 17.0 liters, and the plasma clearance (CLp/F) was calculated to be 14.4 ± 4.5 liters per hour. The plasma level decline was biphasic, with a mean elimination half-life of 3.39 hours (range 1.5 to 12.7 hours). A delayed gastrointestinal absorption (Tmax about 3 hours) of methylergonovine maleate tablet might be observed in postpartum women during continuous treatment with this oxytocic agent.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
General
This drug should not be administered I.V. routinely because of the possibility of inducing sudden hypertensive and cerebrovascular accidents. If I.V. administration is considered essential as a lifesaving measure, methylergonovine maleate should be given slowly over a period of no less than 60 seconds with careful monitoring of blood pressure. Intra-arterial or periarterial injection should be strictly avoided.
Caution should be exercised in the presence of impaired hepatic or renal function.
Breast-feeding
Mothers should not breast-feed during treatment with Methylergonovine Maleate Injection. Milk secreted during this period should be discarded. Methylergonovine Maleate Injection may produce adverse effects in the breast-feeding infant. Methylergonovine Maleate Injection may also reduce the yield of breast milk. Mothers should wait at least 12 hours after administration of the last dose of Methylergonovine Maleate Injection before initiating or resuming breast-feeding.
Coronary artery disease
Patients with coronary artery disease or risk factors for coronary artery disease (e.g., smoking, obesity, diabetes, high cholesterol) may be more susceptible to developing myocardial ischemia and infarction associated with methylergonovine-induced vasospasm.
Medication errors
Inadvertent administration of Methylergonovine Maleate Injection to newborn infants has been reported. In these cases of inadvertent neonatal exposure, symptoms such as respiratory depression, convulsions, cyanosis and oliguria have been reported. Usual treatment is symptomatic. However, in severe cases, respiratory and cardiovascular support is required.
Methylergonovine Maleate Injection has been administered instead of vitamin K and Hepatitis B vaccine, medications which are routinely administered to the newborn. Due to the potential for accidental neonatal exposure, Methylergonovine Maleate Injections should be stored separately from medications intended for neonatal administration.
-
PRECAUTIONS
General
Caution should be exercised in the presence of sepsis, obliterative vascular disease. Also use with caution during the second stage of labor. The necessity for manual removal of a retained placenta should occur only rarely with proper technique and adequate allowance of time for its spontaneous separation.
Drug Interactions
CYP 3A4 Inhibitors (e.g., Macrolide Antibiotics and Protease Inhibitors)
There have been rare reports of serious adverse events in connection with the coadministration of certain ergot alkaloid drugs (e.g., dihydroergotamine and ergotamine) and potent CYP 3A4 inhibitors, resulting in vasospasm leading to cerebral ischemia and/or ischemia of the extremities. Although there have been no reports of such interactions with methylergonovine alone, potent CYP 3A4 inhibitors should not be coadministered with methylergonovine. Examples of some of the more potent CYP 3A4 inhibitors include macrolide antibiotics (e.g., erythromycin, troleandomycin, clarithromycin), HIV protease or reverse transcriptase inhibitors (e.g., ritonavir, indinavir, nelfinavir, delavirdine) or azole antifungals (e.g., ketoconazole, itraconazole, voriconazole).
Less potent CYP 3A4 inhibitors should be administered with caution. Less potent inhibitors include saquinavir, nefazodone, fluconazole, grapefruit juice, fluoxetine, fluvoxamine, zileuton, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP 3A4 of other agents being considered for concomitant use with methylergonovine.
CYP 3A4 inducers
Drugs (e.g., nevirapine, rifampicin) that are strong inducers of CYP 3A4 are likely to decrease the pharmacological action of Methylergonovine Maleate Injection.
Beta-blockers
Caution should be exercised when Methylergonovine Maleate Injection is used concurrently with beta-blockers. Concomitant administration with beta-blockers may enhance the vasoconstrictive action of ergot alkaloids.
Anesthetics
Anesthetics like halothan and methoxyfluran may reduce the oxytocic potency of Methylergonovine Maleate Injection.
Glyceryl trinitrate and other antianginal drugs
Methylergonovine maleate produces vasoconstriction and can be expected to reduce the effect of glyceryl trinitrate and other antianginal drugs.
No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
Caution should be exercised when Methylergonovine Maleate Injection is used concurrently with other vasoconstrictors, ergot alkaloids, or prostaglandins.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed in animals to evaluate carcinogenic potential. The effect of the drug on mutagenesis or fertility has not been determined.
Pregnancy
Category C
Animal reproductive studies have not been conducted with methylergonovine maleate. It is also not known whether methylergonovine maleate can cause fetal harm or can affect reproductive capacity. Use of methylergonovine maleate is contraindicated during pregnancy because of its uterotonic effects. (See INDICATIONS AND USAGE.)
Labor and Delivery
The uterotonic effect of methylergonovine maleate is utilized after delivery to assist involution and decrease hemorrhage, shortening the third stage of labor.
Nursing Mothers
Mothers should not breast-feed during treatment with Methylergonovine Maleate Injection and for at least 12 hours after administration of the last dose. Milk secreted during this period should be discarded.
Geriatric Use
Clinical studies of methylergonovine maleate did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
The most common adverse reaction is hypertension associated in several cases with seizure and/or headache. Hypotension has also been reported. Abdominal pain (caused by uterine contractions), nausea and vomiting have occurred occasionally. Rarely observed reactions have included: acute myocardial infarction, transient chest pains, vasoconstriction, vasospasm, coronary arterial spasm, bradycardia, tachycardia, dyspnea, hematuria, thrombophlebitis, water intoxication, hallucinations, leg cramps, dizziness, tinnitus, nasal congestion, diarrhea, diaphoresis, palpitation, rash and foul taste.
There have been rare isolated reports of anaphylaxis, without a proven causal relationship to the drug product.
Postmarketing Experience
The following adverse drug reactions have been derived from post-marketing experience with Methylergonovine Maleate Injection via spontaneous case reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency, which is therefore categorized as not known.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Symptoms of acute overdose may include: nausea, vomiting, oliguria, abdominal pain, numbness, tingling of the extremities, and rise in blood pressure, in severe cases followed by hypotension, respiratory depression, hypothermia, convulsions, and coma.
Because reports of overdosage with methylergonovine maleate are infrequent, the lethal dose in humans has not been established. The oral LD50 (in mg/kg) for the mouse is 187, the rat 93, and the rabbit 4.5. Several cases of accidental Methylergonovine Maleate Injection in newborn infants have been reported, and in such cases 0.2 mg represents an overdose of great magnitude. However, recovery occurred in all but one case following a period of respiratory depression, hypothermia, hypertonicity with jerking movements, and convulsions.
Also, several children 1-3 years of age have accidentally ingested up to 10 tablets (2 mg) with no apparent ill effects. A postpartum patient took 4 tablets at one time in error and reported paraesthesias and clamminess as her only symptoms.
Treatment of acute overdosage is symptomatic and includes the usual procedures of:
1. removal of offending drug by inducing emesis, gastric lavage, catharsis, and supportive diuresis.
2. maintenance of adequate pulmonary ventilation, especially if convulsions or coma develop.
3. correction of hypotension with pressor drugs as needed.
4. control of convulsions with standard anticonvulsant agents.
5. control of peripheral vasospasm with warmth to the extremities if needed.
-
DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Intramuscularly
1 mL, 0.2 mg, after delivery of the anterior shoulder, after delivery of the placenta, or during the puerperium. May be repeated as required, at intervals of 2-4 hours.
Intravenously
1 mL, 0.2 mg, administered slowly over a period of no less than 60 seconds. (See WARNINGS.)
-
HOW SUPPLIED
Ampules
1 mL size Boxes of 10 NDC: 63629-2074-1 Store and Dispense
Ampules
Store in refrigerator, 2°C-8°C (36°F-46°F). Protect from light. Administer only if solution is clear and colorless.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METHYLERGONOVINE MALEATE
methylergonovine maleate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63629-2074(NDC:51991-144) Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLERGONOVINE MALEATE (UNII: IR84JPZ1RK) (METHYLERGONOVINE - UNII:W53L6FE61V) METHYLERGONOVINE MALEATE 0.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength MALEIC ACID (UNII: 91XW058U2C) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63629-2074-1 10 in 1 BOX 11/07/2016 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040889 11/07/2016 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-2074) , RELABEL(63629-2074)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.