VP-GSTN by Virtus Pharmaceuticals VP-GSTN
VP-GSTN by
Drug Labeling and Warnings
VP-GSTN by is a Other medication manufactured, distributed, or labeled by Virtus Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VP-GSTN- cholecalciferol, genistein, and zinc glycinate citrate capsule
Virtus Pharmaceuticals
----------
VP-GSTN
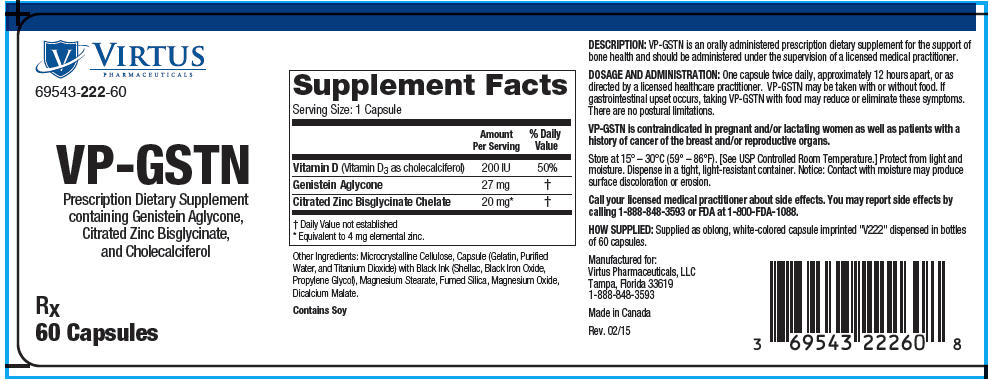
| Supplement Facts
Serving Size: 1 Capsule |
||
|---|---|---|
| Amount Per Serving | % Daily Value | |
|
|
||
|
Vitamin D (Vitamin D3 as cholecalciferol) |
200 IU |
50% |
|
Genistein Aglycone |
27 mg | |
|
Citrated Zinc Bisglycinate Chelate |
20 mg† | |
Other Ingredients: Microcrystalline Cellulose, Capsule (Gelatin, Purified Water, and Titanium Dioxide) with Black Ink (Shellac, Black Iron Oxide, Propylene Glycol), Magnesium Stearate, Fumed Silica, Magnesium Oxide, Dicalcium Malate.
Contains Soy
DESCRIPTION
VP-GSTN is an orally administered prescription dietary supplement for the support of bone health and should be administered under the supervision of a licensed medical practitioner.
DOSAGE AND ADMINISTRATION
One capsule twice daily, approximately 12 hours apart, or as directed by a licensed healthcare practitioner. VP-GSTN may be taken with or without food. If gastrointestinal upset occurs, taking VP-GSTN with food may reduce or eliminate these symptoms. There are no postural limitations.
VP-GSTN is contraindicated in pregnant and/or lactating women as well as patients with a history of cancer of the breast and/or reproductive organs.
Store at 15° – 30°C (59° – 86°F). [See USP Controlled Room Temperature.] Protect from light and moisture. Dispense in a tight, light-resistant container. Notice: Contact with moisture may produce surface discoloration or erosion.
Call your licensed medical practitioner about side effects. You may report side effects by calling 1-888-848-3593 or FDA at 1-800-FDA-1088.
HOW SUPPLIED
Supplied as oblong, white-colored capsule imprinted "V222" dispensed in bottles of 60 capsules.
| VP-GSTN
cholecalciferol, genistein, and zinc glycinate citrate capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| size (solid drugs) | 15 mm | |
| shape | ||
| scoring | 1 | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals (079659493) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.