HALOPERIDOL DECANOATE injection
Haloperidol Decanoate by
Drug Labeling and Warnings
Haloperidol Decanoate by is a Prescription medication manufactured, distributed, or labeled by Lifestar Pharma LLC, Mankind Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HALOPERIDOL DECANOATE safely and effectively. See full prescribing information for HALOPERIDOL DECANOATE.
Haloperidol Decanoate Injection, for intramuscular use
Initial U.S. Approval: 1986WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. HALOPERIDOL DECANOATE INJECTION is not approved for the treatment of patients with dementia-related psychosis (5.1).
INDICATIONS AND USAGE
Haloperidol decanoate injection is a typical antipsychotic indicated for the treatment of schizophrenia in adults who were previously taking a stable dosage of an immediate-release oral haloperidol product. (1)
DOSAGE AND ADMINISTRATION
- Administer haloperidol decanoate by deep intramuscular injection every 4 weeks by a healthcare provider. Do not administer intravenously (2.1).
- For the recommended dosage, including the first recommended dose and the maintenance dosage, see the Dosage and Administration section (2.1).
- If schizophrenia symptoms worsen during dosage modification of haloperidol decanoate, consider administering an immediate-release oral haloperidol product in addition to haloperidol decanoate injection therapy (2.2).
DOSAGE FORMS AND STRENGTHS
Injection: (3)
- Haloperidol 50 mg/mL (present as haloperidol decanoate) in single-dose vials.
- Haloperidol 100 mg/mL (present as haloperidol decanoate) in single-dose vials.
CONTRAINDICATIONS
- Severe toxic central nervous system depression or comatose states from any cause (4).
- Known hypersensitivity to haloperidol or any components of haloperidol decanoate (4).
- Parkinson's disease (4, 5.7, 6.1).
- Dementia with Lewy bodies (4, 5.7).
WARNINGS AND PRECAUTIONS
- Sudden Death, Torsades de Pointes (TdP), and QTc Interval Prolongation:Avoid use of haloperidol decanoate in patients who are at risk of developing TdP. Avoid concomitant use of haloperidol decanoate with drugs that may increase risk of QTc interval prolongation or increase haloperidol exposure. Obtain ECG and serum electrolytes at baseline and during treatment as clinically indicated (5.2).
- Tachycardia and Hypotension:Monitor orthostatic vital signs (5.3).
- Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis:Use with caution in patients with schizophrenia who have risk factors for cerebrovascular adverse reactions (5.4).
- Tardive Dyskinesia:Discontinue treatment if clinically appropriate (5.5).
- Neuroleptic Malignant Syndrome (NMS):Immediately discontinue and monitor closely (5.6).
- Seizures:Haloperidol decanoate injection is generally not recommended in patients receiving antiseizure drugs or who have a history of seizures or EEG abnormalities. If clinically, indicated, maintain patients taking haloperidol decanoate on adequate antiseizure therapy (5.8).
- Potential for Cognitive and Motor Impairment: Advise patients to not drive a motor vehicle or operate hazardous machinery until they are reasonably certain haloperidol decanoate does not impair their cognitive and motor functions (5.11).
- Risk of Encephalopathic Syndrome with Concomitant Use of Lithium:
- Monitor closely for early signs of neurological toxicity and discontinue haloperidol decanoate if such signs appear (5.12).
- Leukopenia, Neutropenia, and Agranulocytosis:Perform complete blood counts (CBC) in patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia. Consider discontinuing haloperidol decanoate if clinically significant decline in WBC occurs in absence of other causative factors. Discontinue haloperidol decanoate in patients with clinically significant neutropenia or an absolute neutrophile count of <1,000/mm3(5.13).
- Hyperprolactinemia: Elevated prolactin levels may occur during acute and chronic use (5.14).
ADVERSE REACTIONS
DRUG INTERACTIONS
- Drugs that Prolong QTc Interval:Avoid concomitant use with haloperidol decanoate (7.1).
- See full prescribing information for additional clinically significant drug interactions with haloperidol decanoate (7.2).
USE IN SPECIFIC POPULATIONS
- Pregnancy:Neonates exposed to haloperidol decanoate during the third trimester of pregnancy may develop extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and decreased feeding) (8.1).
- Lactation: Monitor breastfed infants for excessive sedation, irritability, poor feeding, abnormal muscle movements, and tremors (8.2).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
2.2 Recommended Supplemental Immediate-release Oral Haloperidol Therapy
2.3 Preparation Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
5.2 Sudden Death, Torsades de Pointes, and QTc Interval Prolongation
5.3 Tachycardia and Hypotension
5.4 Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis
5.5 Tardive Dyskinesia
5.6 Neuroleptic Malignant Syndrome
5.7 Neurological Adverse Reactions in Patients with Parkinson’s Disease or Dementia with Lewy Bodies
5.8 Seizures
5.9 Hypersensitivity Reactions
5.10 Falls
5.11 Potential for Cognitive and Motor Impairment
5.12 Risk of Encephalopathic Syndrome with Concomitant Use of Lithium
5.13 Leukopenia, Neutropenia, and Agranulocytosis
5.14 Hyperprolactinemia
5.15 Risk of Severe Neurotoxicity in Patients with Thyrotoxicosis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs that Prolong the QTc Interval
7.2 Other Clinically Significant Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Haloperidol decanoate injection is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
Administer haloperidol decanoate by deep intramuscular injection every 4 weeks by a health care professional. Do not administer haloperidol decanoate intravenously.
When injecting haloperidol decanoate, use a 21-gauge needle. The maximum volume per injection site is 3 mL.
Table 1 below describes the recommended dosage for haloperidol decanoate. The maximum recommended initial dose is 100 mg. If the calculated first recommended dose of haloperidol decanoate injection is greater than 100 mg, then administer two deep intramuscular injections as follows:
100 mg on the first day
Remainder of the amount 3 to 7 days later.
Table 1: Haloperidol Decanoate Recommended Dosage
Population
First Recommended Dose
Maintenance Dosage1
Adult patients less than 65 years old stabilized on ≤ 10 mg of daily immediate-release oral haloperidol with normal hepatic function.
10-15 times previous daily dose of immediate-release oral haloperidol.
10-15 times previous daily dose of immediate-release oral haloperidol administered every 4 weeks.
The dosage may be increased by increments of 50 mg or less every 4 weeks until an optimal therapeutic effect is obtained.
The typical effective dosage range is between 50 and 200 mg every 4 weeks.
Adult patients less than 65 years old stabilized on ≤ 10 mg of daily immediate-release oral haloperidol with hepatic impairment OR
Adult patients 65 years and older
10-15 times previous daily dose of immediate-release oral haloperidol. Consider starting at the low end of the dosing range [see Use in Specific Populations (8.5, 8.6) and Clinical Pharmacology (12.3)].
Adult patients less than 65 years old stabilized on >10 mg of daily immediate-release oral haloperidol
10-20 times previous daily dose of immediate-release oral haloperidol
10-15 times previous daily dose of immediate-release oral haloperidol administered every 4 weeks. The dosage may be increased by increments of 50 mg or less every 4 weeks until an optimal therapeutic effect is obtained.
1Clinical experience with haloperidol decanoate at a dosage greater than 450 mg every 4 weeks has been limited.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Haloperidol decanoate injection is contraindicated in patients with:
- Severe toxic central nervous system depression or comatose states from any cause.
- Known hypersensitivity to haloperidol or any components of haloperidol decanoate. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported in patients treated with haloperidol [see Warnings and Precautions (5.9) and Adverse Reactions (6.2)].
- Parkinson's disease [see Warnings and Precautions (5.7)].
- Dementia with Lewy bodies [see Warnings and Precautions (5.7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. In an analysis of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, the risk of death in antipsychotic drug-treated patients was 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the incidence of death in antipsychotic drug-treated patients was about 4.5%, compared to an incidence of about 2.6% in placebo-treated patients. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature.
Haloperidol decanoate injection is not approved for the treatment of patients with dementia-related psychosis [see Indications and Usage (1)].
5.2 Sudden Death, Torsades de Pointes, and QTc Interval Prolongation
Cases of sudden death, torsades de pointes (TdP) and QTc interval prolongation have been reported in haloperidol-treated patients [see Adverse Reactions (6.1, 6.2)]. Cases have been reported even in the absence of predisposing factors. Higher than recommended haloperidol dosages were associated with a higher risk of TdP and QTc interval prolongation.
Avoid use of haloperidol decanoate in patients who are at significant risk of developing TdP including those with congenital long QT syndrome, uncontrolled or significant cardiac disease, recent myocardial infarction, ischemic cardiomyopathy, unstable angina, bradyarrhythmias, uncontrolled hypertension, high degree atrioventricular block, severe aortic stenosis, or uncontrolled hypothyroidism. Avoid the concomitant use of haloperidol decanoate with drugs that may increase the risk of the QTc interval prolongation or increase haloperidol exposure. Assess the QTc interval via an ECG at baseline, and during treatment as clinically indicated. Obtain serum electrolytes (including potassium, calcium, phosphorus, and magnesium) at baseline and during treatment as clinically indicated, and correct electrolyte abnormalities.
5.3 Tachycardia and Hypotension
Tachycardia and hypotension (including orthostatic hypotension) have been reported in patients treated with haloperidol [see Adverse Reactions (6.1)]. Orthostatic vital signs should be monitored in patients who are at risk for hypotension (e.g., geriatric patients, patients with dehydration, hypovolemia, and concomitantly treated with antihypertensive medications), patients with known cardiovascular disease (history of myocardial infarction, ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease.
Should hypotension occur and a vasopressor be required, epinephrine must not be used since haloperidol decanoate may block its vasopressor activity, and paradoxically lower blood pressure.
Instead, metaraminol, phenylephrine or norepinephrine should be used.
5.4 Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials, elderly patients with dementia-related psychosis treated with antipsychotics had an increased risk of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack) including fatalities, compared to those treated with placebo. The mechanism for this increased risk is not known.
Haloperidol decanoate injection is not approved for the treatment of patients with dementia-related psychosis. Haloperidol decanoate should be used with caution in patients with schizophrenia who have risk factors for cerebrovascular adverse reactions.
5.5 Tardive Dyskinesia
Tardive dyskinesia (TD) may develop in patients treated with antipsychotic drugs, including haloperidol decanoate [see Adverse Reactions (6.1)]. TD can develop after a relatively brief treatment period at low dosages and may also occur after discontinuation of treatment. If antipsychotic treatment is discontinued, TD may partially or completely remit. Antipsychotic treatment, however, may suppress or partially suppress the signs and symptoms of TD and may mask the underlying process. The effect that symptomatic suppression has upon the long-term course of TD is unknown.
The TD risk in patients treated with antipsychotic drugs appears to be highest among the elderly, especially elderly women, but it is not possible to predict, which patients are likely to develop TD. The TD risk and the likelihood that TD will become irreversible increase with the duration of antipsychotic drug treatment and the cumulative dosage. In patients who require chronic antipsychotic treatment, use the lowest dosage and the shortest duration of treatment that produces a satisfactory clinical response. Periodically reassess the need for continued treatment. If signs and symptoms of TD appear in haloperidol decanoate-treated patients, consider drug discontinuation. However, some patients may require haloperidol decanoate treatment despite the presence of TD.
5.6 Neuroleptic Malignant Syndrome
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex, has been reported in association with the use of antipsychotic drugs [see Adverse Reactions (6.1)]. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, delirium, and autonomic instability, and additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
If NMS is suspected, immediately discontinue haloperidol decanoate and provide intensive symptomatic treatment and monitoring.
5.7 Neurological Adverse Reactions in Patients with Parkinson’s Disease or Dementia with Lewy Bodies
Patients with Parkinson's disease or Dementia with Lewy bodies may experience increased sensitivity to haloperidol. Manifestations of this increased sensitivity include severe extrapyramidal symptoms (e.g., tremor, rigidity, bradykinesia), confusion, sedation, and falls. Haloperidol decanoate injection is contraindicated in patients with Dementia with Lewy bodies and in patients with Parkinson's disease.
5.8 Seizures
Haloperidol decanoate may lower the seizure threshold. Haloperidol decanoate injection is generally not recommended in patients receiving antiseizure drugs or have a history of seizures or EEG abnormalities. If clinically indicated, maintain patients taking haloperidol decanoate on adequate antiseizure therapy.
5.9 Hypersensitivity Reactions
There have been postmarketing reports of hypersensitivity reactions with haloperidol including anaphylactic reaction, angioedema, dermatitis exfoliative, hypersensitivity vasculitis, rash, urticaria, face edema, laryngeal edema, bronchospasm, and laryngospasm [see Adverse Reactions (6.2)].
Haloperidol decanoate injection is contraindicated in patients with known hypersensitivity to haloperidol or any components of haloperidol decanoate.
5.10 Falls
Antipsychotics, including haloperidol decanoate, may cause somnolence, orthostatic hypotension, motor instability and sensory abnormality, which may lead to falls and, consequently, fractures and other injuries.
If patients have a condition (or take concomitant drugs) that could exacerbate these effects, complete fall risk assessments when initiating haloperidol decanoate treatment and periodically during long-term treatment.
5.11 Potential for Cognitive and Motor Impairment
Haloperidol decanoate may impair judgement, thinking, or motor skills. Inform patients of the risk and advise them to not drive a motor vehicle or operate hazardous machinery until they are reasonably certain that treatment with haloperidol decanoate does not impair their cognitive and motor functions.
5.12 Risk of Encephalopathic Syndrome with Concomitant Use of Lithium
An encephalopathic syndrome, characterized by weakness, lethargy, fever, tremulousness, confusion, extrapyramidal symptoms, leukocytosis, and elevated serum enzymes (AST, ALT, GGT, alkaline phosphatase, CK, and LDH), BUN, and fasting blood sugar, followed by irreversible brain damage has occurred in a few patients treated with concomitant haloperidol and lithium.
Monitor patients who concomitantly use haloperidol decanoate and lithium closely for early signs of neurological toxicity, and discontinue haloperidol decanoate or both haloperidol decanoate and lithium promptly if such signs appear.
5.13 Leukopenia, Neutropenia, and Agranulocytosis
Leukopenia, neutropenia and agranulocytosis (including fatal cases) have been reported during treatment with antipsychotic drugs, including haloperidol decanoate [see Adverse Reactions (6.2)].
Possible risk factors for antipsychotic drug-associated leukopenia and neutropenia include pre-existing low WBC and history of drug-induced leukopenia and neutropenia.
Perform frequent complete blood count (CBC) monitoring during the first few months of haloperidol decanoate therapy in patients with a history of a clinically significant low WBC, drug-induced leukopenia or neutropenia. Consider discontinuing haloperidol decanoate in patients who have a clinically significant decline in their WBC in the absence of other causative factors. Discontinue haloperidol decanoate in patients with clinically significant neutropenia or an absolute neutrophil count of <1,000/mm3 and monitor closely until the neutropenia resolves.
5.14 Hyperprolactinemia
Antipsychotic drugs elevate prolactin levels during acute and chronic use and may result in galactorrhea, amenorrhea, gynecomastia, and impotence which have been reported with antipsychotic drugs [see Adverse Reactions (6.1, 6.2) and Use in Specific Populations (8.3)].
Published epidemiologic studies have shown inconsistent results regarding the potential association between hyperprolactinemia and breast cancer [see Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Sudden Death, Torsades de Pointes, and QTc Interval Prolongation [see Warnings and Precautions (5.2)]
- Tachycardia and Hypotension [see Warnings and Precautions (5.3)]
- Tardive Dyskinesia [see Warnings and Precautions (5.5)]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.6)]
- Seizures [see Warnings and Precautions (5.8)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.9)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.13)]
- Hyperprolactinemia [see Warnings and Precautions (5.14)]
- Risk of Severe Neurotoxicity in Patients with Thyrotoxicosis [see Warnings and Precautions (5.15)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions Identified in Clinical Trials with haloperidol decanoate
The data described below reflect exposure to 15 mg to 500 mg (1.7 times the maximum recommended dosage) of haloperidol decanoate monthly in 13 clinical trials of 410 adult patients with schizophrenia or an unapproved condition. These clinical trials comprised of:
- 1 double-blind, active comparator-controlled trial with fluphenazine decanoate (Trial 1).
- 2 trials comparing haloperidol decanoate to oral haloperidol (Trials 2 and 3).
- 9 open-label trials.
- 1 dose-response trial.
The most common adverse reactions that occurred in ≥5% of haloperidol decanoate-treated patients in Trial 1 were Parkinsonism and oculogyric crisis.
Adverse reactions that occurred in ≥1% of haloperidol decanoate-treated patients in Trial 1 are shown in Table 2. Trial 1 was not designed to evaluate meaningful comparisons of the incidence of adverse reactions in the haloperidol decanoate and fluphenazine decanoate treatment groups.
Table 2: Adverse Reactions that Occurred in ≥1% of haloperidol decanoate-treated Patients and Fluphenazine Decanoate-treated Patients in Trial 1a
Haloperidol decanoate (n=36)
Fluphenazine decanoate (n=36)
Extrapyramidal disorder:
Parkinsonism
31%
44%
Oculogyric crisis
6%
0%
Akinesia
3%
22%
Akathisia
3%
14%
Tremor
3%
0%
Abdominal pain
3%
0%
Headache
3%
0%
a The study was not designed to evaluate meaningful comparisons of the incidence of adverse reactions in the haloperidol decanoate and the fluphenazine decanoate treatment groups.
Less common adverse reactions (<1%) that occurred in Trial 1 and other adverse reactions that occurred in Trials 2 and 3, and open-label and dose-response clinical trials of haloperidol decanoate are listed below.
- Cardiac Disorders: Tachycardia
- Endocrine Disorders: Hyperprolactinemia
- Eye Disorders: Vision blurred
- Gastrointestinal Disorders: Constipation, Dry mouth, Salivary hypersecretion
- General Disorders and Administration Site Conditions: Weight increased, Injection site reaction
- Musculoskeletal and Connective Tissue Disorders: Muscle rigidity
- Nervous System Disorders: Dyskinesia, Dystonia, Cogwheel rigidity, Hypertonia, Masked facies, Sedation, Somnolence
- Reproductive System Disorders: Erectile dysfunction
Adverse Reactions Identified in Clinical Trials with Immediate-Release Haloperidol Products
Based on clinical trials with immediate-release haloperidol products that included 1,579 patients, the following adverse reactions were reported:
- Musculoskeletal and Connective Tissue Disorders: Torticollis, Trismus, Muscle twitching
- Nervous System Disorders: Neuroleptic malignant syndrome, Tardive dyskinesia, Bradykinesia, Hyperkinesia, Hypokinesia, Dizziness, Nystagmus
- Psychiatric Disorders: Loss of libido, Restlessness
- Reproductive System and Breast Disorders: Amenorrhea, Galactorrhea, Dysmenorrhea, Menorrhagia, Breast discomfort
- Skin and Subcutaneous Tissue Disorders: Acneiform skin reactions
- Vascular Disorders: Hypotension, Orthostatic hypotension
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of haloperidol, including haloperidol decanoate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Pancytopenia, Agranulocytosis, Thrombocytopenia, Leukopenia, Neutropenia
- Cardiac Disorders: Ventricular fibrillation, Torsade de pointes, Ventricular tachycardia, Extrasystoles, QTc interval prolongation
- Endocrine Disorders: Inappropriate antidiuretic hormone secretion
- Gastrointestinal Disorders: Vomiting, Nausea
- General Disorders and Administration Site Conditions: Sudden death, Face edema, Edema, Hyperthermia, Hypothermia, Injection site abscess, Weight decreased
- Hepatobiliary Disorders: Acute hepatic failure, Hepatitis, Cholestasis, Jaundice, Liver function test abnormal
- Immune System Disorders: Anaphylactic reaction, Hypersensitivity
- Metabolic and Nutritional Disorders: Hypoglycemia
- Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis
- Nervous System Disorders: Convulsion, Opisthotonus, Tardive dystonia
- Pregnancy, Puerperium and Perinatal Conditions: Neonatal drug withdrawal syndrome
- Psychiatric Disorders: Agitation, Confusional state, Depression, Insomnia
- Renal and Urinary Disorders: Urinary retention
- Reproductive System and Breast Disorders: Priapism, Gynecomastia
- Respiratory, Thoracic and Mediastinal Disorders: Laryngeal edema, Bronchospasm, Laryngospasm, Dyspnea
- Skin and Subcutaneous Tissue Disorders: Angioedema, Dermatitis exfoliative, Hypersensitivity vasculitis, Photosensitivity reaction, Urticaria, Pruritus, Rash, Hyperhidrosis
-
7 DRUG INTERACTIONS
7.1 Drugs that Prolong the QTc Interval
Avoid concomitant use of haloperidol decanoate with other drugs with a known potential to prolong the QTc interval. If concomitant use cannot be avoided [see Warnings and Precautions (5.2)]:
- Obtain ECGs when initiating and during concomitant use as clinically indicated.
- Obtain serum electrolytes (including potassium, calcium, phosphorus, and magnesium) when initiating and during concomitant use as clinically indicated.
QTc interval prolongation has been observed with haloperidol decanoate treatment. Concomitant use of haloperidol decanoate with other products that prolong the QTc interval may result in a greater increase in the QTc interval, and adverse reactions associated with QTc interval prolongation, including torsade de pointes, other serious arrythmias, and sudden death [see Warnings and Precautions (5.2)].
7.2 Other Clinically Significant Drug Interactions
Table 3 describes other clinically significant drug interactions of haloperidol decanoate.
Table 3: Other Clinically Significant Drugs Interactions1
CNS Depressants
Clinical Impact
Haloperidol may potentiate CNS depressants.
Prevention or Management
Avoid concomitant use of haloperidol decanoate with CNS depressants such as anesthetics, opioids, and alcohol.
CYP3A4 and/or CYP2D6 Inhibitors
Clinical Impact
CYP3A4 and/or CYP2D6 inhibitors increase haloperidol exposure (haloperidol is a CYP3A4 and CYP2D6 substrate) [see Clinical Pharmacology (12.3)]. Concomitant use of haloperidol decanoate and CYP3A4 and/or CYP2D6 inhibitors may increase the risk of haloperidol-associated adverse reactions.
Prevention or Management
Monitor for signs or symptoms of increased or prolonged pharmacologic effects of haloperidol. Decrease the dosage of haloperidol decanoate as clinically necessary.
CYP3A4 Inducers
Clinical Impact
CYP3A4 inducers decrease haloperidol exposure (haloperidol is a CYP3A4 substrate) [see Clinical Pharmacology (12.3)] . Concomitant use of haloperidol decanoate with CYP3A4 inducers may reduce the effectiveness of haloperidol decanoate.
Prevention or Management
Monitor patients and if necessary, increase the dosage of haloperidol decanoate.
CYP2D6 Substrates
Clinical Impact
Haloperidol is a CYP2D6 inhibitor. Plasma concentrations of CYP2D6 substrates may increase when they are concomitantly administered with haloperidol decanoate.
Prevention or Management
Monitor plasma concentrations of the CYP2D6 substrate, if possible. Consider reducing the dosage of the CYP2D6 substrate, if necessary. Refer to the Prescribing Information of the CYP2D6 substrate.
Dopaminergic Drugs
Clinical Impact
Haloperidol may antagonize the effects of levodopa, dopamine agonists, and other drugs intended to increase dopamine levels.
Prevention or Management
Haloperidol decanoate injection is contraindicated in patients with Parkinson's disease and dementia with Lewy bodies. For conditions other than Parkinson's disease and dementia with Lewy bodies, when possible, avoid concomitant use of haloperidol decanoate with dopaminergic drugs [see Warnings and Precautions (5.7)] .
Anticholinergic Drugs
Clinical Impact
The healthcare provider should keep in mind the possible increase in intraocular pressure when anticholinergic drugs are administered concomitantly with haloperidol decanoate.
Prevention or Management
Monitor and manage patients as clinically appropriate.
Lithium
Clinical Impact
Concomitant use of haloperidol decanoate with lithium may cause an encephalopathic syndrome followed by irreversible brain damage [see Warnings and Precautions (5.12)] .
Prevention or Management
Monitor patients who concomitantly use haloperidol decanoate with lithium closely for early signs of neurological toxicity, and discontinue haloperidol decanoate or both haloperidol decanoate and lithium promptly if such signs appear.
1See www.fda.gov/CYPandTransporterInteractingDrugs for examples of CYP3A4 and/or CYP2D6 inhibitors, CYP3A4 inducers, and CYP2D6 substrates.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Available data from published epidemiologic studies of pregnant patients exposed to haloperidol have not established a drug-associated risk of major birth defects or miscarriage. Case reports of limb malformations in neonates have been reported in haloperidol-treated mothers; however, causal relationships were not established in these cases. There are risks to the pregnant patient from untreated schizophrenia, including increased risk of relapse, hospitalization, and suicide (see Clinical Considerations). Haloperidol decanoate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and decreased feeding) following delivery (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage in patients with schizophrenia is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk: There is risk to the pregnant patient from untreated schizophrenia, including increased risk of schizophrenia relapse, hospitalization, and suicide. Schizophrenia is associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/Neonatal Adverse Reactions: Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and decreased feeding have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. Transient neonatal dyskinesia has also been reported. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization. Monitor neonates for extrapyramidal, withdrawal, and dyskinesia symptoms and manage symptoms appropriately.
Data
Animal Data:
Rats or rabbits administered oral haloperidol at doses of 0.5 to 7.5 mg/kg (approximately 0.2 to 7 times the maximum recommended human oral dose (MRHD) of 20 mg/day based on mg/m2body surface area) showed an increase in incidence of resorption, reduced fertility, delayed delivery, and pup mortality. No fetal abnormalities were observed at these doses in rats or rabbits. Cleft palate has been observed in mice administered oral haloperidol at a dose of 0.5 mg/kg, which is approximately 0.1 times the oral MRHD based on mg/m2body surface area.
8.2 Lactation
Literature reports suggest that haloperidol is detected in human milk of haloperidol- treated mothers with a relative infant dose ranging from 2% to 12%. Haloperidol has also been detected in the plasma and urine of breastfed infants. There has been a report of lethargy, poor feeding, and slowing of motor movements in an infant exposed to haloperidol through human milk. Haloperidol may increase prolactin levels in some patients which can lead to galactorrhea.
Monitor infants exposed to haloperidol decanoate via human milk for excessive sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors and abnormal muscle movements).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for haloperidol decanoate and any potential adverse effects on the breastfed child from haloperidol decanoate or from the mother's underlying condition.
8.3 Females and Males of Reproductive Potential
Females: Based on the pharmacologic action of haloperidol (D2 receptor antagonism), treatment with haloperidol decanoate may result in an increase in serum prolactin levels, which may lead to a reduction in fertility in females of reproductive potential.
Males: Based on animal studies, male fertility may be impaired by treatment with haloperidol [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of haloperidol decanoate have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of haloperidol decanoate did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
The exposure of haloperidol may be higher in geriatric patients compared to young adult patients. Results from small clinical studies suggest a lower clearance and a longer elimination half-life of haloperidol in geriatric patients [see Clinical Pharmacology (12.3)]. Consider starting at the low end of the recommended dosing range for the first haloperidol decanoate dose in geriatric patients [see Dosage and Administration (2.1)].
Antipsychotic drugs increase the risk of death in elderly patients with dementia-related psychosis. Haloperidol decanoate injection is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
Elderly patients with dementia-related psychosis treated with antipsychotics had an increased risk of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack) including fatalities, compared to those treated with placebo [see Warnings and Precautions (5.4)].
Antipsychotic drugs increase the risk of tardive dyskinesia and this risk appears to be highest among the elderly, particularly elderly women [see Warnings and Precautions (5.5)].
8.6 Hepatic Impairment
Haloperidol is extensively metabolized in the liver, therefore, haloperidol concentrations may be higher in patients with hepatic impairment compared to patients with normal hepatic function. In patients with hepatic impairment, consider starting at the low end of the recommended dosing range for the first haloperidol decanoate dose [see Dosage and Administration (2.1)].
-
10 OVERDOSAGE
Reported overdose signs and symptoms are those resulting from an exaggeration of the drug's known pharmacologic effects (e.g., drowsiness and sedation, tachycardia and hypotension) and adverse reactions, the most prominent of which would be: 1) severe extrapyramidal symptoms, 2) hypotension, or 3) sedation. Patients may appear comatose with respiratory depression and hypotension which could be severe enough to produce a shock-like state. Extrapyramidal reactions may be manifested by muscular weakness or rigidity and a generalized or localized tremor, as demonstrated by the akinetic or agitans types, respectively. The risk of QTc interval prolongation and torsade de pointes should be considered [see Adverse Reactions (6.2)].
Management of Overdose
There is no specific antidote for a haloperidol overdose.
- Should hypotension occur and a vasopressor be required, epinephrine must not be used since haloperidol decanoate may block its vasopressor activity, and paradoxical further lowering of the blood pressure may occur. Instead, metaraminol, phenylephrine or norepinephrine should be used.
- In case of severe extrapyramidal reactions, antiparkinson drugs should be administered, and should be continued for several weeks, and then withdrawn gradually as extrapyramidal symptoms may emerge if discontinued abruptly.
- Monitor ECG and vital signs for signs of QTc interval prolongation or dysrhythmias and continue monitoring until the dysrhythmias resolve and the haloperidol-induced QTc interval prolongation resolves.
- Dialysis is not recommended in the treatment of overdose because it removes only very small amounts of haloperidol.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
Haloperidol decanoate injection is the decanoate ester of haloperidol, for intramuscular use. Haloperidol is a typical antipsychotic. The structural formula of haloperidol decanoate 4-(4-Chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-piperidin-4-yl decanoate, is:
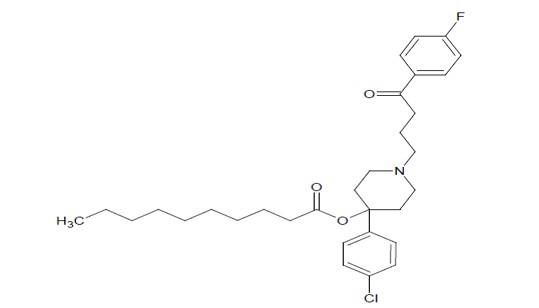
The molecular formula is C31H41CIFNO3 and has a molecular weight of 530.12. Haloperidol decanoate, USP is almost insoluble in water (0.01 mg/mL), but is soluble in most organic solvents. Each mL of haloperidol decanoate injection contains:
- 50 mg of haloperidol (present as 70.5 mg of haloperidol decanoate) in a sesame oil vehicle with 1.2% (w/v) benzyl alcohol as a preservative.
- 100 mg of haloperidol (present as 141 mg of haloperidol decanoate) in a sesame oil vehicle with 1.2% (w/v) benzyl alcohol as a preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of haloperidol decanoate for the treatment of schizophrenia in adults is unclear. However, its effect in schizophrenia could be mediated through its activity as an antagonist at central dopamine type 2 receptors. Haloperidol also binds to alpha-1 adrenergic receptors, but with lower affinity, and displays minimal binding to muscarinic cholinergic and histaminergic (H1) receptors.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of haloperidol have not been fully characterized.
12.3 Pharmacokinetics
The plasma concentrations of haloperidol gradually rise, reaching a peak at about 6 days after the haloperidol decanoate injection, and fall thereafter, with an apparent half-life of about 3 weeks. Steady state plasma concentrations of haloperidol are achieved within 2 to 4 months in patients receiving monthly haloperidol decanoate injections.
The relationship between the haloperidol decanoate dosage and plasma haloperidol concentration is roughly linear for dosages below 450 mg (1.5 times the maximum recommended dosage [see Dosage and Administration (2.1)]; however, the pharmacokinetics of haloperidol following intramuscular injections can be quite variable between patients.
Elimination
Metabolism:
Haloperidol is metabolized by several routes. The major pathways are glucuronidation and ketone reduction. The cytochrome P450 enzyme system is also involved, particularly CYP3A4 and, to a lesser extent, CYP2D6.
Excretion:
Less than 3% of administered haloperidol is eliminated unchanged in the urine. The apparent half-life of haloperidol following intramuscular injection of haloperidol decanoate is about 3 weeks.
Specific Populations
Patients with Hepatic Impairment
Studies in patients with hepatic impairment have not been conducted. Haloperidol is extensively metabolized in the liver, therefore, haloperidol concentrations may be higher in patients with hepatic impairment compared to patients with normal hepatic function [see Use in Specific Populations (8.6)].
Geriatric Patients
Haloperidol plasma concentrations in geriatric patients were higher than in younger adult patients when administered the same dosage. Results from small clinical studies suggest a lower clearance and a longer elimination half-life of haloperidol in geriatric patients. The results are within the observed variability in haloperidol pharmacokinetics [see Use in Specific Populations (8.5)].
Patients with Renal Impairment
Studies in patients with renal impairment have not been conducted.
Drug Interaction Studies
Ketoconazole and Paroxetine
The haloperidol plasma concentrations increased when ketoconazole (400 mg/day, strong CYP3A4 inhibitor) and paroxetine (20 mg/day, strong CYP2D6 inhibitor) were concomitantly administered with haloperidol [see Drug Interactions (7.2)].
Valproate: Sodium valproate, a drug known to inhibit glucuronidation, does not affect haloperidol plasma concentrations.
Rifampin: In a study with 12 patients with schizophrenia, concomitant administration of oral haloperidol and rifampin, a strong CYP3A4 inducer, resulted in decreased plasma haloperidol concentrations by a mean of 70% and increased mean scores on the Brief Psychiatric Rating Scale from baseline. In five other patients with schizophrenia treated with oral haloperidol and rifampin, discontinuation of rifampin resulted in a mean 3.3-fold increase in haloperidol concentrations [see Drug Interactions (7.2)].
Carbamazepine: In a study with 11 patients with schizophrenia, concomitant administration of haloperidol and increasing doses of carbamazepine, a CYP3A4 strong inducer, resulted in decreased haloperidol plasma concentrations in a linear manner with increasing carbamazepine concentrations [see Drug Interactions (7.2)].
Effect of Haloperidol on Other Drugs
Haloperidol is an inhibitor of CYP2D6. Plasma concentrations of CYP2D6 substrates may increase when they are concomitantly administered with haloperidol [see Drug Interactions (7.2)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenicity studies using oral haloperidol were conducted in Wistar rats (dosed at up to 5 mg/kg daily for 24 months) and in Albino Swiss mice (dosed at up to 5 mg/kg daily for 18 months).
- In the rat study, survival was reduced in all haloperidol dose groups, decreasing the number of rats at risk for developing tumors. However, a relatively greater number of rats survived to the end of the study in the high dose haloperidol male and female groups. These haloperidol-treated rats at doses up to approximately 2.5 times the maximum recommended human oral dose (MRHD) of haloperidol of 20 mg/day based on mg/m2body surface area did not have a greater incidence of tumors than control-treated rats.
- In female mice, there was a statistically significant increase in mammary gland neoplasia and total tumor incidence at haloperidol doses approximately 0.3 and 1.2 times the oral MRHD based on mg/m2body surface area and there was statistically significant increase in pituitary gland neoplasia at approximately 1.2 times the oral MRHD. In male mice, no statistically significant differences in incidences of total tumors or specific tumor types were noted.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. An increase in mammary neoplasms has been found in rodents after chronic administration of antipsychotic drugs [see Warnings and Precautions (5.14)].
Mutagenesis
No mutagenic potential of haloperidol decanoate was found in the Ames Salmonella assay. Negative or inconsistent positive findings have been obtained in in vitro and in vivo studies of effects of haloperidol on chromosome structure and number. The available cytogenetic evidence is considered too inconsistent to be conclusive.
Impairment of Fertility
Haloperidol was orally administered to male rats at doses of 0.5, 2.5, and 15 mg/kg/day (approximately 0.2 to 7 times the oral MRHD based on mg/m2 body surface area) for 63 days prior to mating with untreated females. Decreases in mating performance and fertility, as well as markedly decreased motor activity, was observed at seven times the oral MRHD based on mg/m2 body surface area. The NOAEL of 2.5 mg/kg/day is approximately equal to the oral MRHD based on mg/m2 body surface area.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Haloperidol decanoate injection, 50 mg/mL is a clear colourless/pink to yellow amber (brown) solution for IM injection containing 50 mg haloperidol as 70.52 mg per mL haloperidol decanoate, USP in:
NDC: 70756-615-81 1 mL single-dose vial in individual carton. Discard unused portion.
NDC: 70756-615-33 1 mL single-dose vials packed in carton of 3's. Discard unused portion.
NDC: 70756-615-10 1 mL single-dose vials packed in carton of 10's. Discard unused portion.
NDC: 70756-624-85 5 mL multi-dose vial in individual carton.
NDC: 70756-624-10 5 mL multi-dose vials packed in carton of 10's.
Haloperidol decanoate injection, 100 mg/mL is a clear colourless/pink to yellow amber (brown) solution for IM injection containing 100 mg haloperidol as 141.04 mg per mL haloperidol decanoate, USP in:
NDC: 70756-616-81 1 mL single-dose vial in individual carton. Discard unused portion.
NDC: 70756-616-05 1 mL single-dose vials packed in carton of 5's. Discard unused portion
NDC: 70756-616-10 1 mL single-dose vials packed in carton of 10's. Discard unused portion
NDC: 70756-625-85 5 mL multi-dose vial in individual carton.
NDC: 70756-625-05 5 mL multi-dose vials packed in carton of 5's.
NDC: 70756-625-10 5 mL multi-dose vials packed in carton of 10's.
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F) excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]. Do not refrigerate or freeze. Protect from light. Discard unused portion.
-
17 PATIENT COUNSELING INFORMATION
Sudden Death, Torsades de Pointes, and QTc Interval Prolongation
Inform patients that there have been reports of sudden death, torsades de Pointes, and QTc interval prolongation in haloperidol-treated patients. Advise patients or caregivers to seek immediate medical attention if they suspect or develop signs or symptoms associated with the clinical consequences of QTc interval prolongation [see Warnings and Precautions (5.2)].
Tardive Dyskinesia
Inform patients that tardive dyskinesia (TD) may develop with haloperidol decanoate. Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their healthcare provider if these abnormal movements occur [see Warnings and Precautions (5.5)].
Neuroleptic Malignant Syndrome
Counsel patients about a potentially fatal adverse reaction, Neuroleptic Malignant Syndrome (NMS), that has been reported with administration of antipsychotic drugs. Advise patients, family members, or caregivers to contact the health care provider or to report to the emergency room if they experience signs and symptoms of NMS [see Warnings and Precautions (5.6)].
Hypersensitivity Reactions
Inform patients of the potential risk of hypersensitivity reactions. Advise patients to stop taking haloperidol decanoate and seek immediate attention if signs or symptoms of a hypersensitivity reaction occur [see Warnings and Precautions (5.9)].
Falls
Inform patients that haloperidol decanoate can cause somnolence, orthostatic hypotension, motor instability and sensory abnormality that may lead to falls. Advise patients to notify their healthcare provider if any of these symptoms occur [see Warnings and Precautions (5.10)].
Potential for Cognitive and Motor Impairment
Inform patients of the risk and advise them to not drive a motor vehicle or operate hazardous machinery until they are reasonably certain that treatment with haloperidol decanoate does not impair their cognitive and motor functions [see Warnings and Precautions (5.11)].
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug induced leukopenia or neutropenia that they should have their CBC monitored while taking haloperidol decanoate [see Warnings and Precautions (5.13)].
Hyperprolactinemia
Counsel patients on signs and symptoms of hyperprolactinemia that may be associated with chronic use of haloperidol decanoate. Advise the patients to seek medical attention if they experience any of the following: amenorrhea, galactorrhea, erectile dysfunction or gynecomastia [see Warnings and Precautions (5.14)].
Pregnancy
Advise pregnant patients to notify their health care provider if they become pregnant or intend to become pregnant during treatment with haloperidol decanoate. Advise patients that haloperidol decanoate exposure during the third trimester of pregnancy may cause adverse effects in the neonate, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and decreased feeding [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding patients using haloperidol decanoate to monitor infants for excess sedation, irritability, poor feeding, and extrapyramidal symptoms (tremors and abnormal muscle movements) and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Infertility
Advise females and males of reproductive potential that haloperidol decanoate may impair fertility due to an increase in serum prolactin levels [see Use in Specific Populations (8.3)].
Drug Interactions
Advise patients to inform their health care provider before starting or discontinuing a prescription drug, nonprescription drug, or supplement [see Warnings and Precautions (5.2) and Drug Interactions (7)].
Manufactured for:
Lifestar Pharma LLC
1200 MacArthur Blvd
Mahwah, NJ 07430 USA
Made in India
Revised: November 2025, V-07
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 70756-615-81
Haloperidol Decanoate
Injection
50 mg/mL
For Intramuscular Use Only
1 mL Single-Dose Vial
Sterile
Rx Only

NDC: 70756-615-81
Haloperidol
Decanoate
Injection
50 mg/mL
For Intramuscular
Use Only
1 mL Single-Dose Vial
Sterile
Rx Only
NDC: 70756-615-33
Haloperidol
Decanoate
Injection
50 mg/mL
For Intramuscular
Use Only
3 x 1 mL Single-Dose Vials
Sterile
Rx Only
NDC: 70756-615-10
Haloperidol
Decanoate
Injection
50 mg/mL
For Intramuscular
Use Only
10 x 1 mL Single-Dose Vials
Sterile
Rx Only
NDC: 70756-624-85
Haloperidol Decanoate
Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular Use Only
5 mL Multi-Dose Vial
Sterile
Rx Only

NDC: 70756-624-85
Haloperidol
Decanoate
Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular
Use Only
5 mL Multi-Dose Vial
Sterile
Rx Only
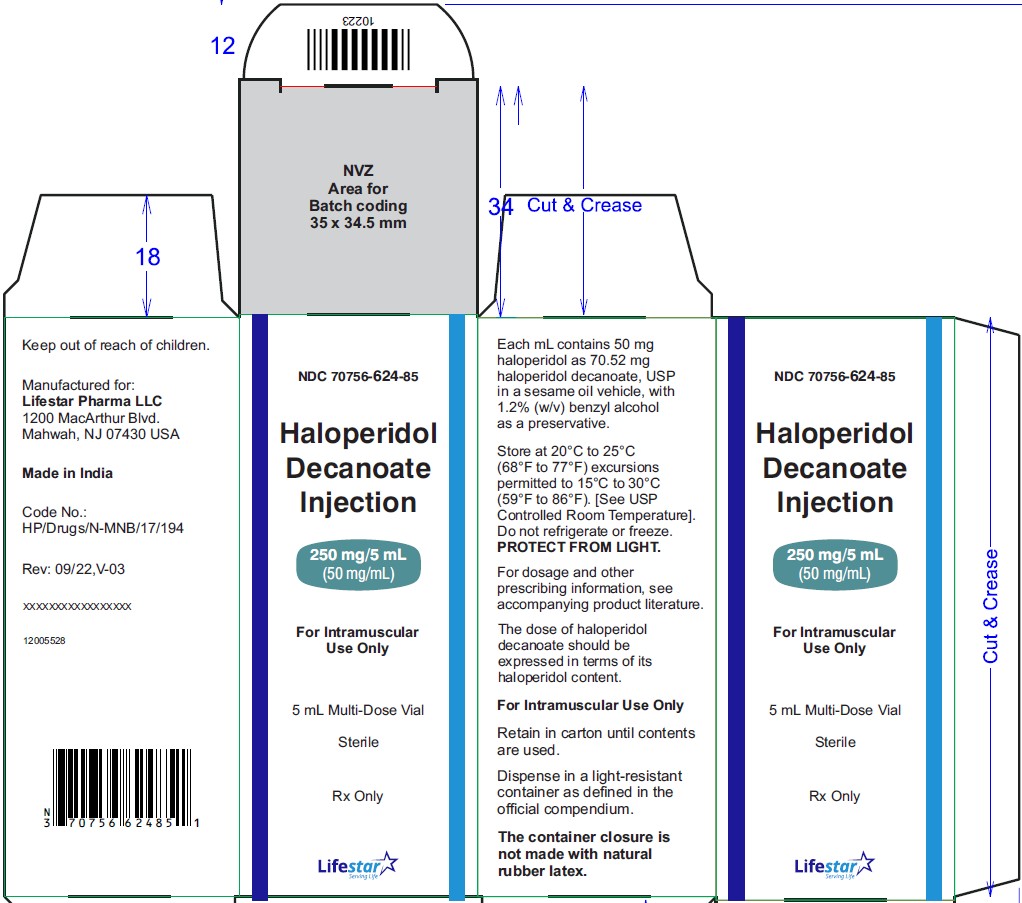
NDC: 70756-624-10
Haloperidol
Decanoate
Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular
Use Only
10 x 5 mL Multi-Dose Vials
Sterile
Rx Only
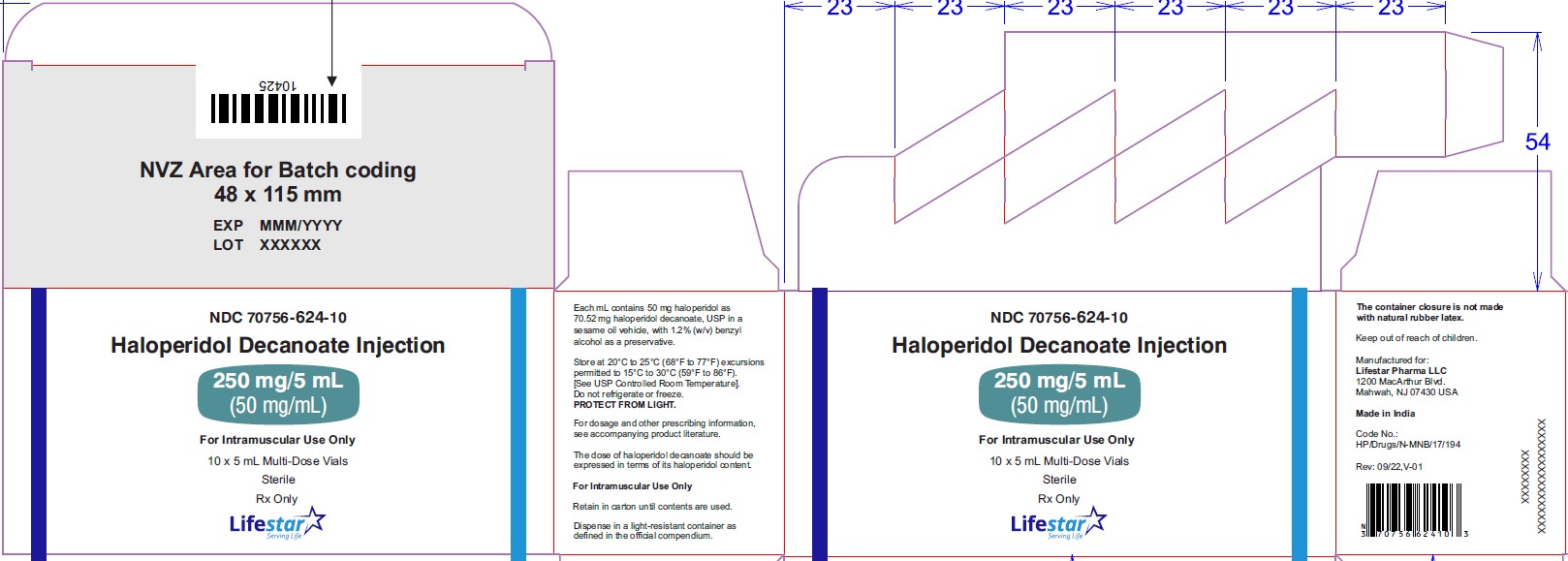
NDC: 70756-616-81
Haloperidol Decanoate
Injection
100 mg/mL
For Intramuscular Use Only
1 mL Single-Dose Vial
Sterile
Rx Only

NDC: 70756-616-81
Haloperidol
Decanoate
Injection
100 mg/mL
For Intramuscular
Use Only
1 mL Single-Dose Vial
Sterile
Rx Only
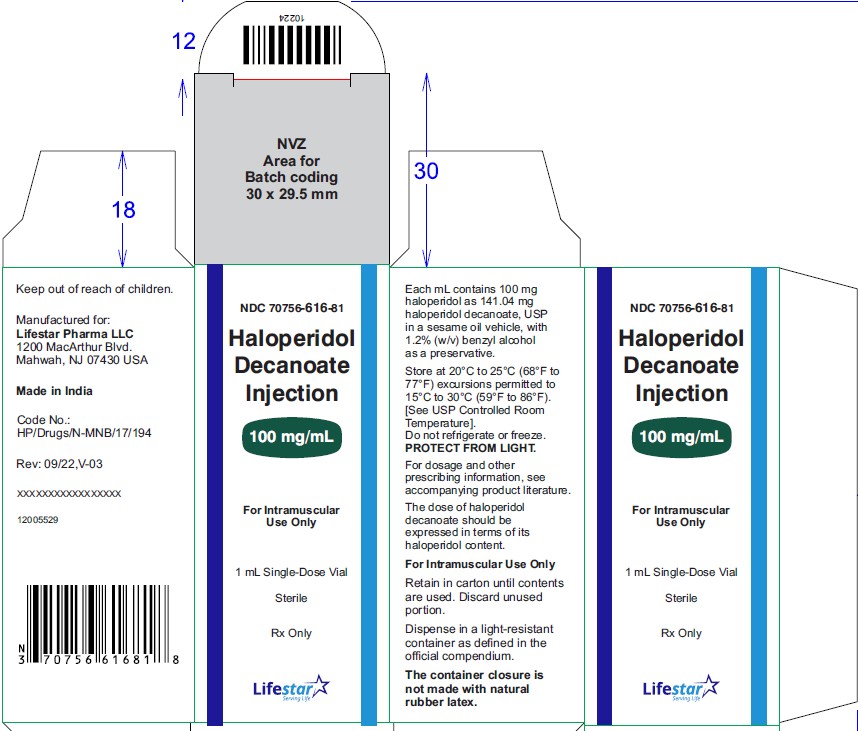
NDC: 70756-616-05
Haloperidol
Decanoate
Injection
100 mg/mL
For Intramuscular
Use Only
5 x 1 mL Single-Dose Vials
Sterile
Rx Only
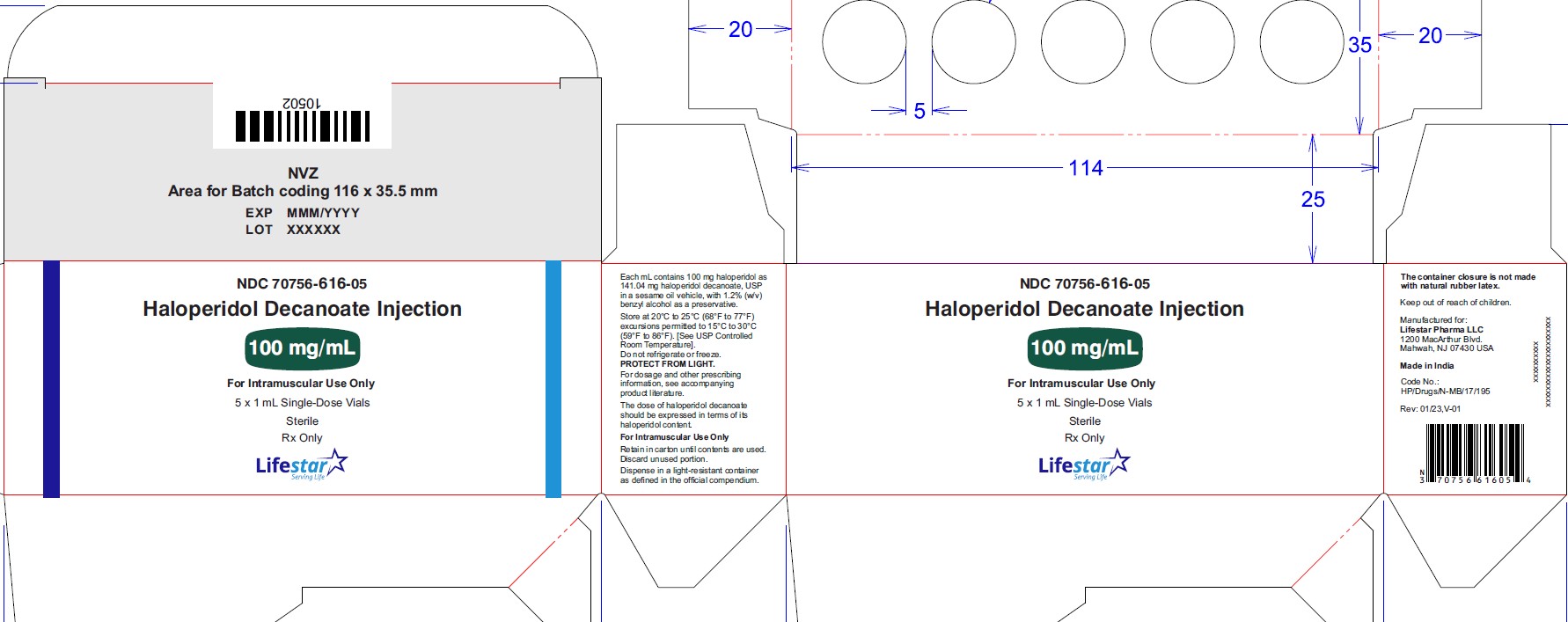
NDC: 70756-616-10
Haloperidol
Decanoate
Injection
100 mg/mL
For Intramuscular
Use Only
10 x 1 mL Single-Dose Vials
Sterile
Rx Only
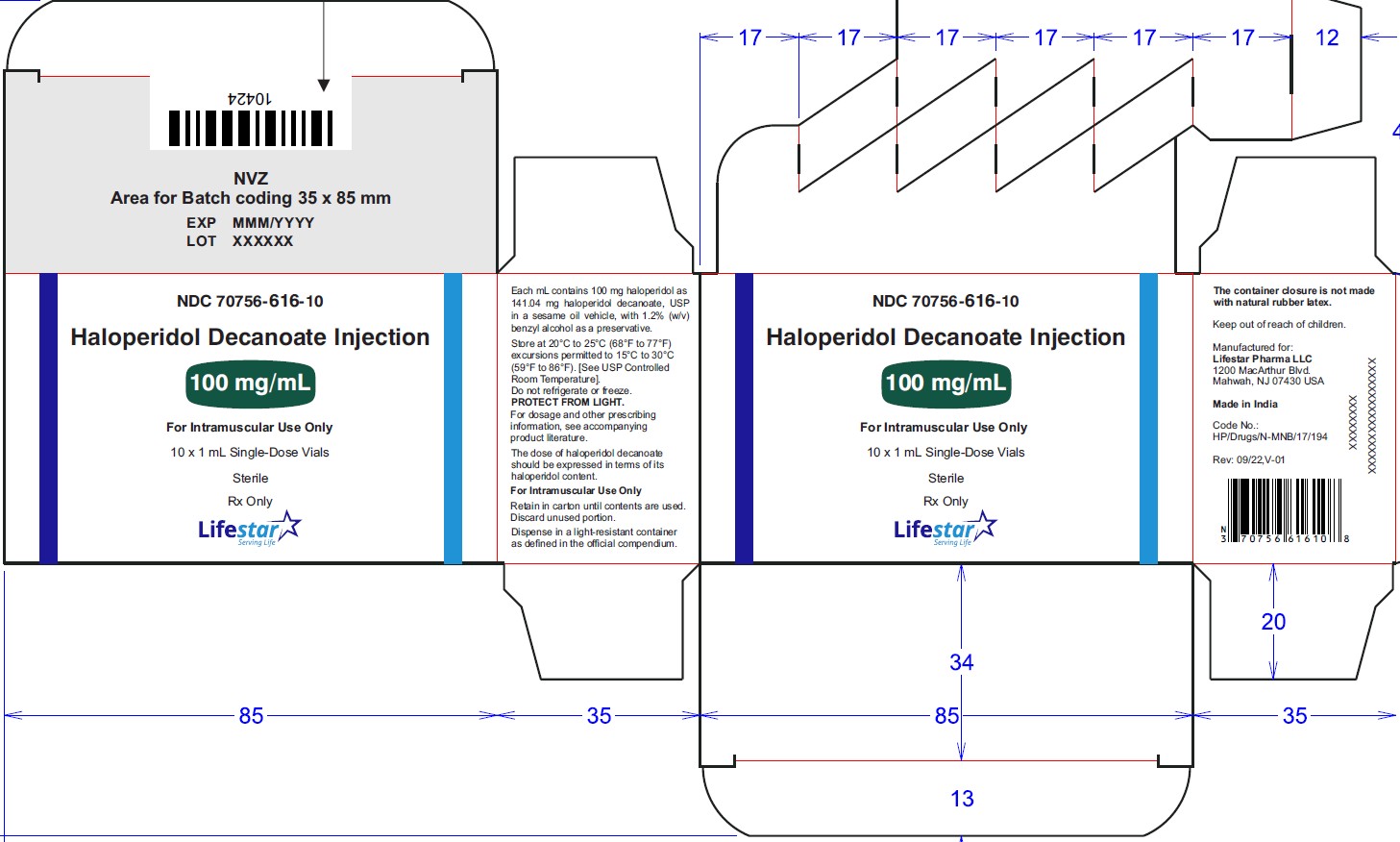
NDC: 70756-625-85
Haloperidol Decanoate
Injection
500 mg/5 mL (100 mg/mL)
For Intramuscular Use Only
5 mL Multi-Dose Vial
Sterile
Rx Only

NDC: 70756-625-85
Haloperidol
Decanoate
Injection
500 mg/5 mL (100 mg/mL)
For Intramuscular
Use Only
5 mL Multi-Dose Vial
Sterile
Rx Only
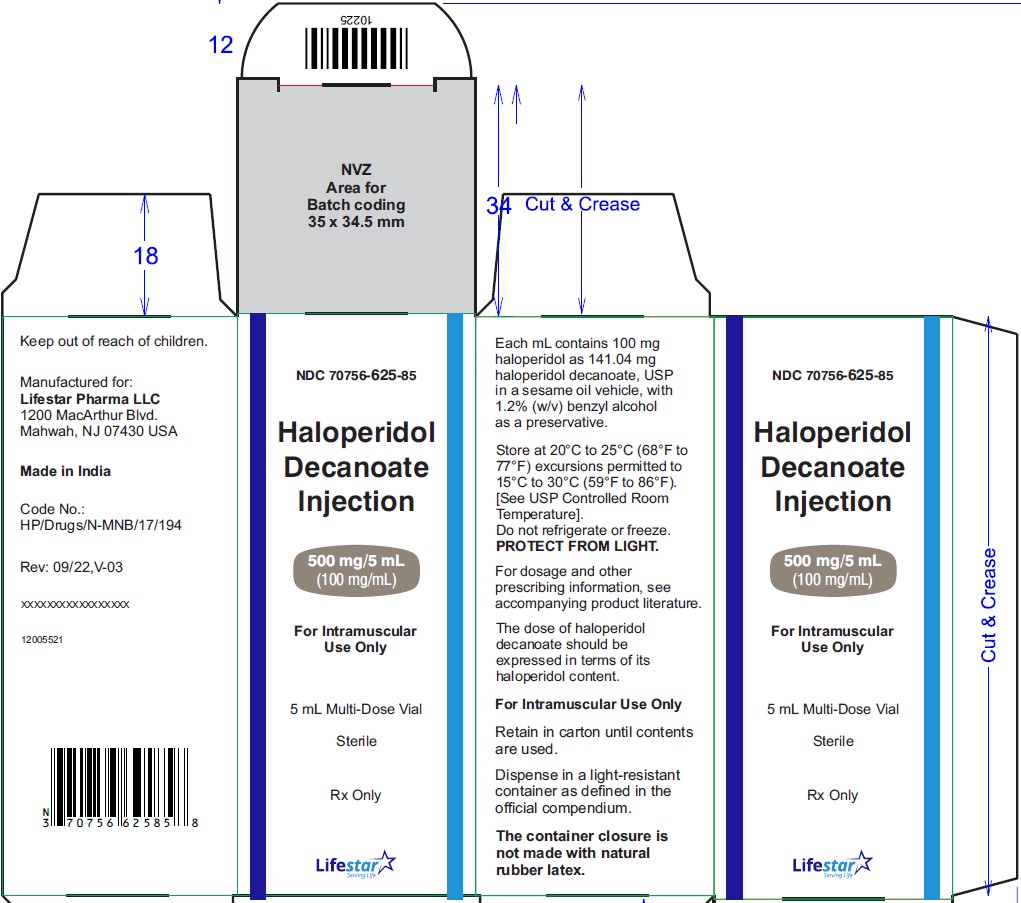
NDC: 70756-625-05
Haloperidol
Decanoate
Injection
500 mg/5 mL (100 mg/mL)
For Intramuscular
Use Only
5 x 5 mL Multi-Dose Vials
Sterile
Rx Only
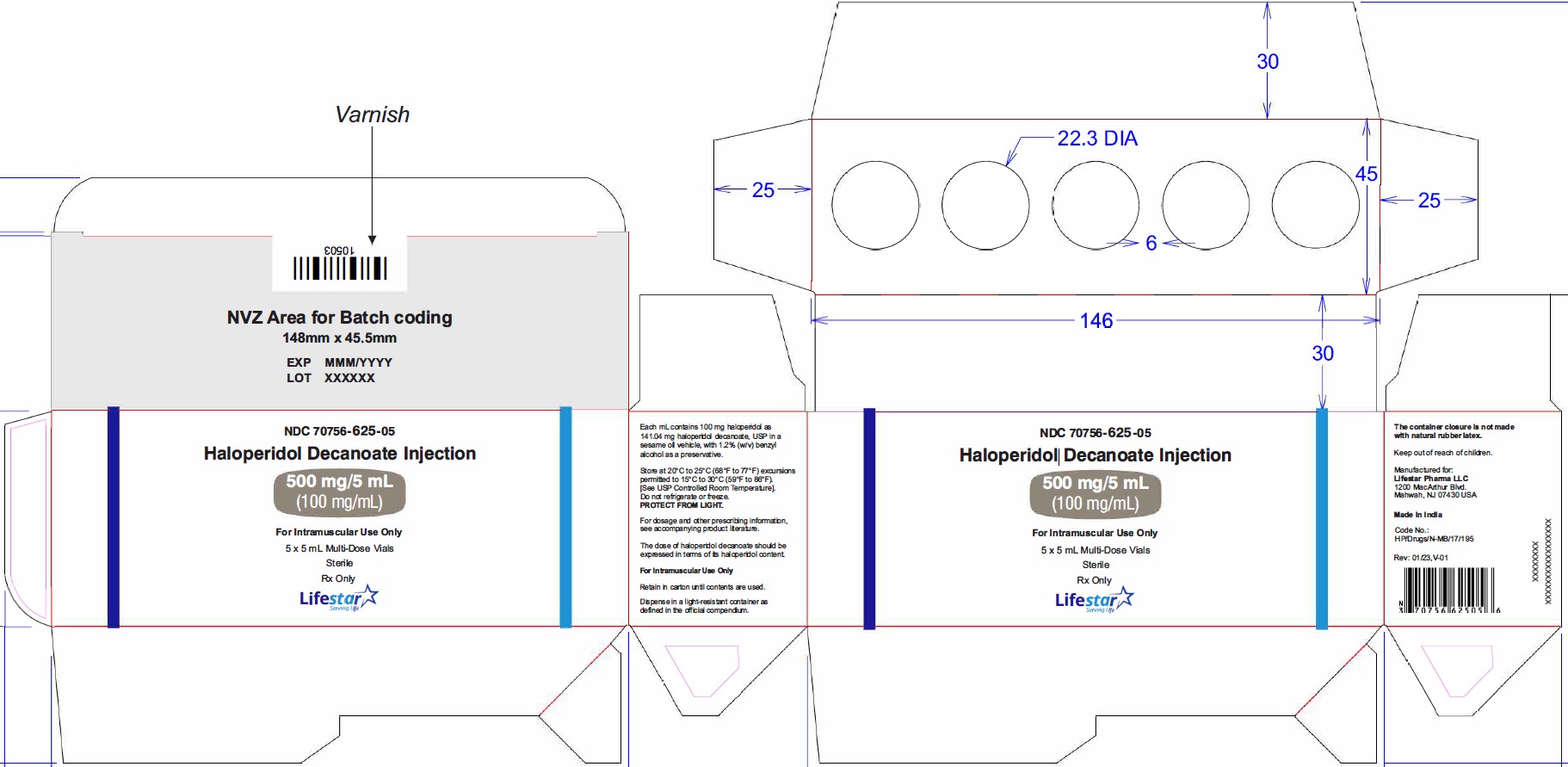
NDC: 70756-625-10
Haloperidol
Decanoate
Injection
500 mg/5 mL (100 mg/mL)
For Intramuscular
Use Only
10 x 5 mL Multi-Dose Vials
Sterile
Rx Only
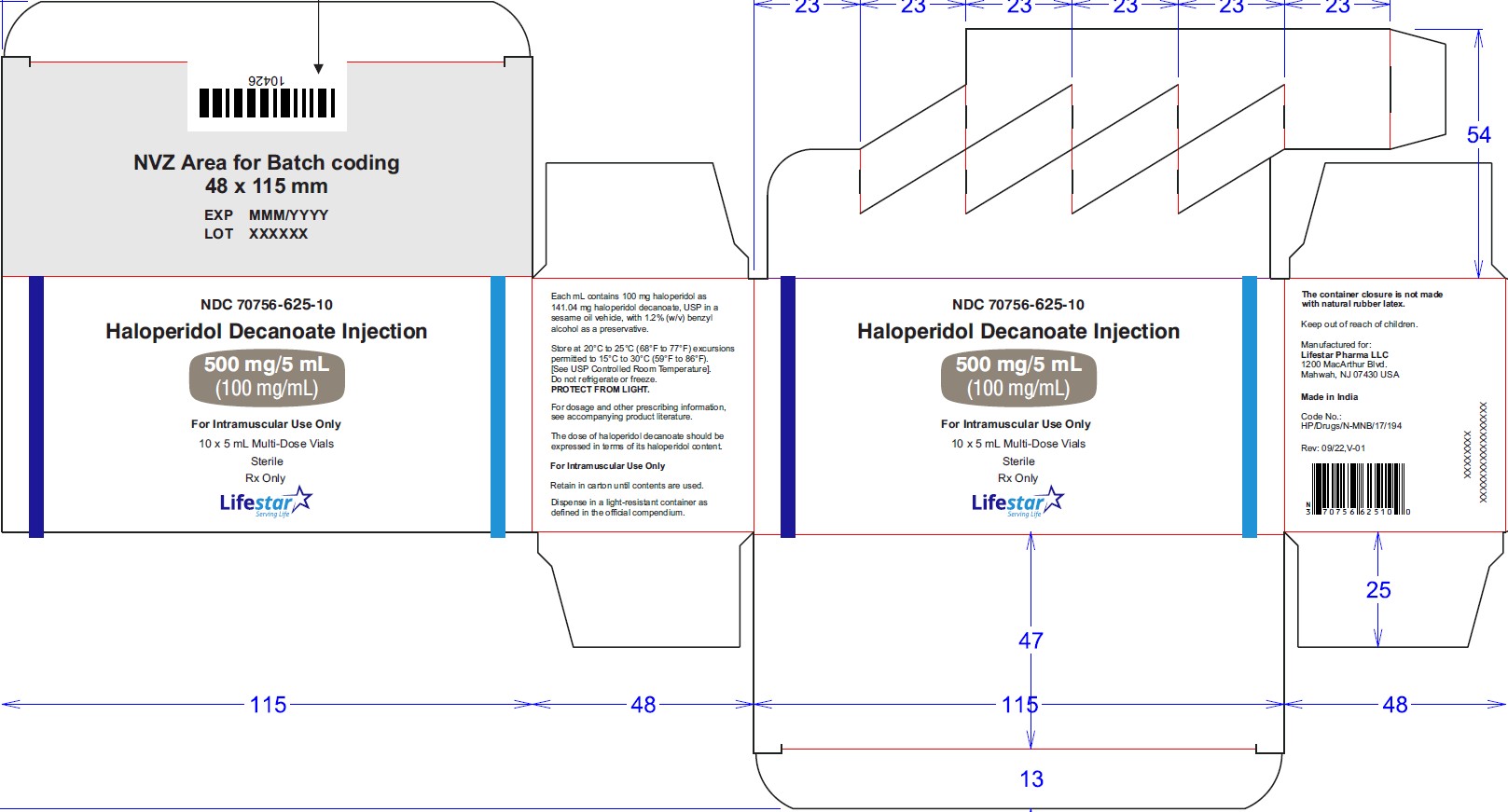
-
INGREDIENTS AND APPEARANCE
HALOPERIDOL DECANOATE
haloperidol decanoate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70756-615 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HALOPERIDOL DECANOATE (UNII: AC20PJ4101) (HALOPERIDOL - UNII:J6292F8L3D) HALOPERIDOL 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SESAME OIL (UNII: QX10HYY4QV) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70756-615-81 1 in 1 CARTON 05/23/2023 1 1 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 70756-615-10 10 in 1 CARTON 05/23/2023 2 1 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 70756-615-33 3 in 1 CARTON 05/23/2023 3 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216730 05/23/2023 HALOPERIDOL DECANOATE
haloperidol decanoate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70756-624 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HALOPERIDOL DECANOATE (UNII: AC20PJ4101) (HALOPERIDOL - UNII:J6292F8L3D) HALOPERIDOL 250 mg in 5 mL Inactive Ingredients Ingredient Name Strength SESAME OIL (UNII: QX10HYY4QV) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70756-624-85 1 in 1 CARTON 05/23/2023 1 5 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 70756-624-10 10 in 1 CARTON 05/23/2023 2 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216730 05/23/2023 HALOPERIDOL DECANOATE
haloperidol decanoate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70756-616 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HALOPERIDOL DECANOATE (UNII: AC20PJ4101) (HALOPERIDOL - UNII:J6292F8L3D) HALOPERIDOL 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength SESAME OIL (UNII: QX10HYY4QV) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70756-616-81 1 in 1 CARTON 05/23/2023 1 1 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 70756-616-05 5 in 1 CARTON 05/23/2023 2 1 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 70756-616-10 10 in 1 CARTON 05/23/2023 3 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216730 05/23/2023 HALOPERIDOL DECANOATE
haloperidol decanoate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70756-625 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HALOPERIDOL DECANOATE (UNII: AC20PJ4101) (HALOPERIDOL - UNII:J6292F8L3D) HALOPERIDOL 500 mg in 5 mL Inactive Ingredients Ingredient Name Strength SESAME OIL (UNII: QX10HYY4QV) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70756-625-85 1 in 1 CARTON 05/23/2023 1 5 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 70756-625-05 5 in 1 CARTON 05/23/2023 2 5 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 70756-625-10 10 in 1 CARTON 05/23/2023 3 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216730 05/23/2023 Labeler - Lifestar Pharma LLC (080268943) Registrant - Mankind Pharma Limited (915834068) Establishment Name Address ID/FEI Business Operations Mankind Pharma Limited 916512493 MANUFACTURE(70756-615, 70756-624, 70756-616, 70756-625) , ANALYSIS(70756-615, 70756-624, 70756-616, 70756-625) , PACK(70756-615, 70756-624, 70756-616, 70756-625)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.