POLY HIST FORTE- doxylamine succinate and phenylephrine hydrochloride tablet
Poly Hist Forte by
Drug Labeling and Warnings
Poly Hist Forte by is a Otc medication manufactured, distributed, or labeled by Poly Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not exceed recommended dosage.
-
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Ask a doctor before use if
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions? Comments?
-
PRINCIPAL DISPLAY PANEL
NDC: 50991-626-01
POLY HIST FORTE ®
TABLETS
Nasal Decongestant Antihistamine
NEW FORMULA
Each tablet contains:
Doxylamine Succinate . . . 10.5 mg
Phenylephrine HCl . . . . . . 10 mg
100 Tablets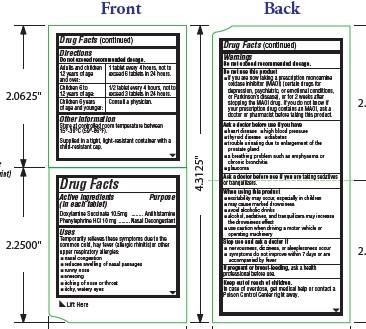
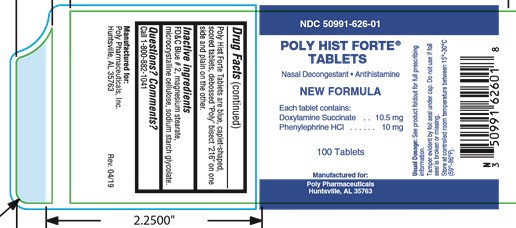
-
INGREDIENTS AND APPEARANCE
POLY HIST FORTE
doxylamine succinate and phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50991-626 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 10.5 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color blue Score 2 pieces Shape CAPSULE Size 14mm Flavor Imprint Code Poly;216 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50991-626-02 12 in 1 CARTON 06/01/2019 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 50991-626-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/01/2019 Labeler - Poly Pharmaceuticals, Inc. (198449894)
Trademark Results [Poly Hist Forte]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 POLY HIST FORTE 76157717 2611825 Live/Registered |
Poly Pharmaceuticals, Inc. 2000-11-02 |
 POLY HIST FORTE 73718740 1550698 Dead/Cancelled |
POLY PHARMACEUTICALS, INC. 1988-03-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.