4379 FIRST AID KIT kit 4334 FIRST AID KIT kit
4334 First Aid Kit by
Drug Labeling and Warnings
4334 First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Honeywell Safety Products USA, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
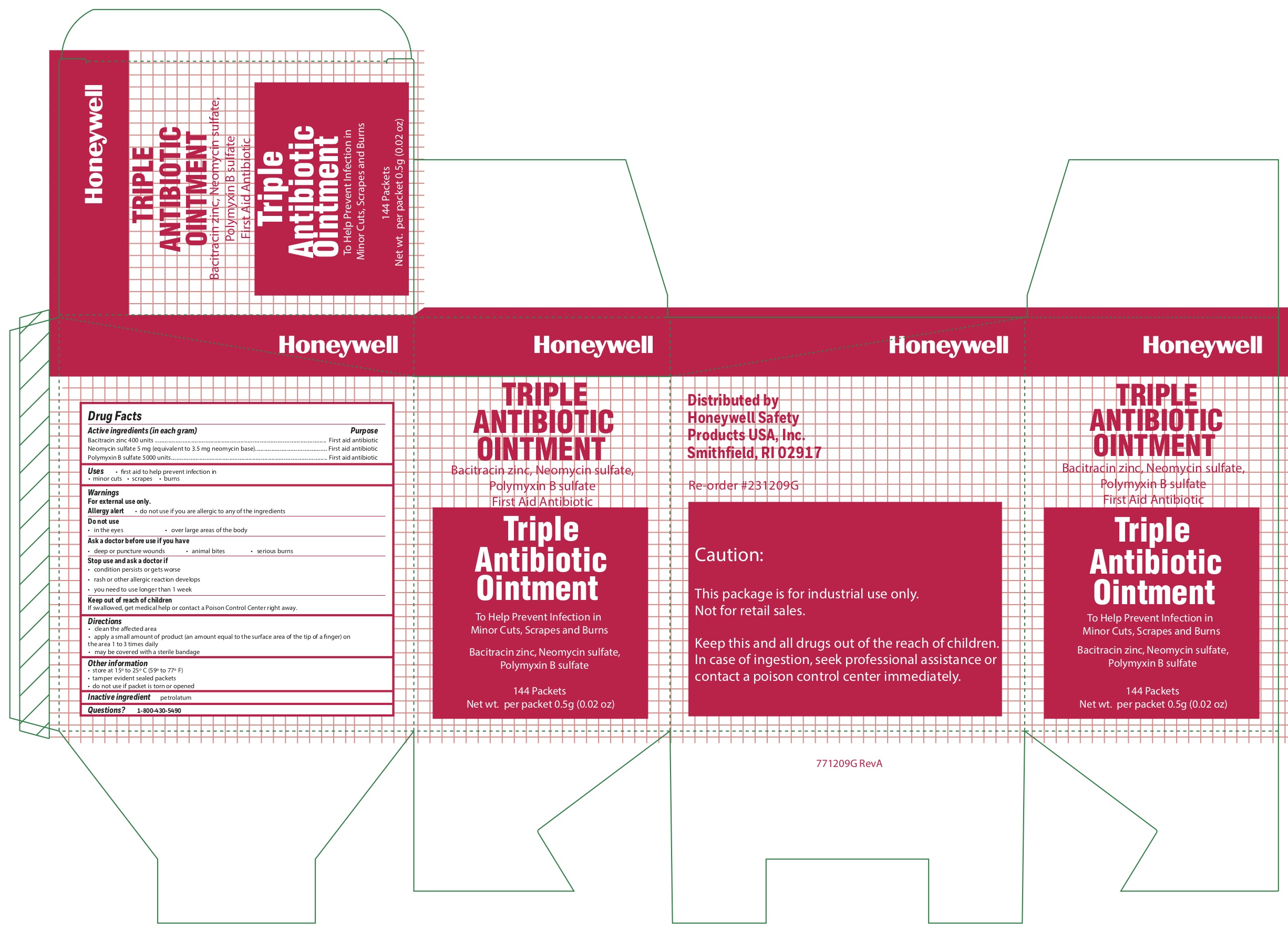
- Triple Active ingredients
- Triple Purpose
- Triple Uses
- Triple Warnings
- Triple Directions
- Triple Other information
- Triple Inactive ingredient
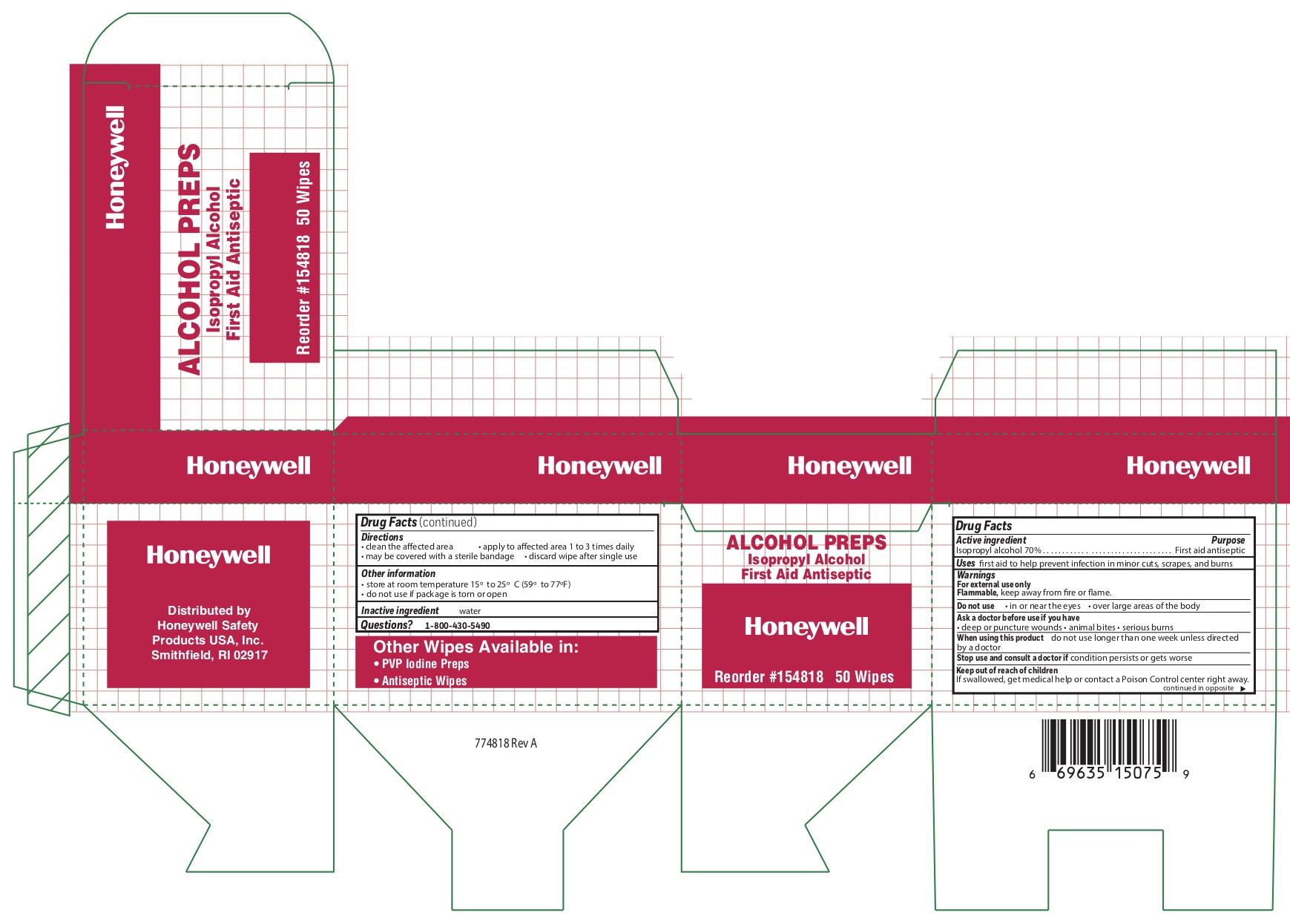
- Alcohol Active ingredient
- Alcohol Purpose
- Alcohol Uses
- Alcohol Warnings
- Alcohol Directions
- Alcohol Other information
- Alcohol Inactive ingredient
- Foille Active ingredient
- Foille Purpose
- Foille Uses
- Foille Warnings
- Foille Directions
- Foille Other information
- Foille Inactive ingredients
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn Jel Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
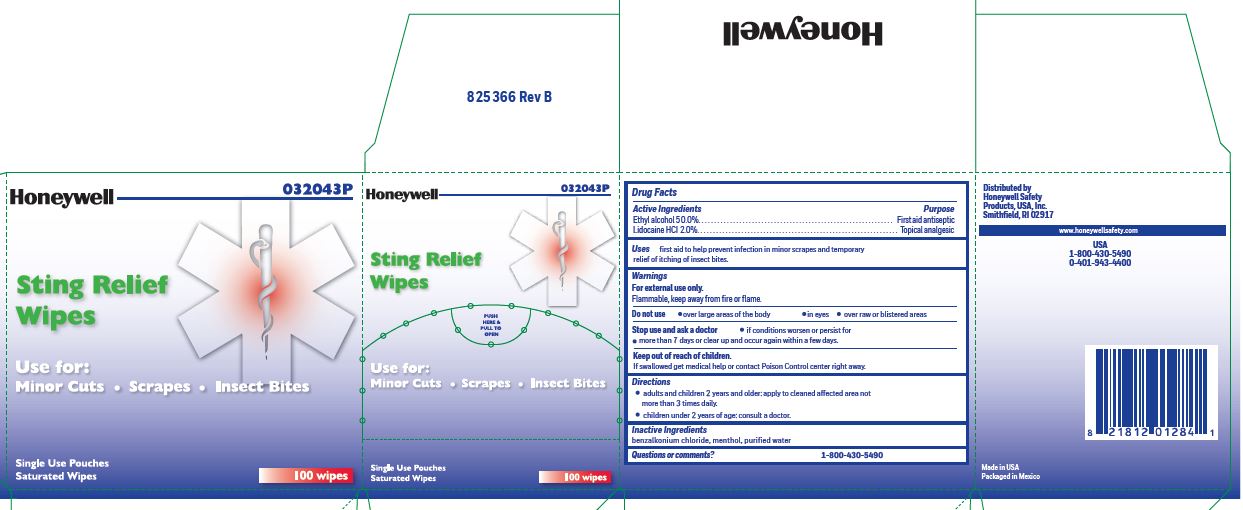
- Sting Relief Active ingredients (in each wipe)
- Sting Relief Purpose
- Sting Relief Uses
- Sting Relief Warnings
- Sting Relief Directions
- Sting Relief Inactive ingredients
- Sting Relief Questions or Comments?
- PVP Wipe Active ingredient
- PVP Purpose
- PVP USes
- PVP Warnings
- PVP Directions
- PVP Other information
- PVP Inactive ingredients
- PVP Questions
- Aypanal EX Active ingredient
- Aypanal EX Purpose
- Aypanal EX Uses
-
Aypanal EX
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away
Do Not Use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
- Aypanal EX Directions
- Aypanal EX Other information
- Aypanal EX Inactive ingredients
- Aypanal EX Questions or Comments?
- BZK Active ingredient
- BZK Purpose
- BZK Uses
-
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
- BZK Directions
- BZK Other information
- BZK Inactive ingredients
- BZK Questions
-
4334
Z019819 KIT CONTENTS
1 TRIPLE ANTIBIOTIC 10 PER
1 INSTANT COLD PACK 4" X 6"
2 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 BURN JEL 1/8 OZ, 6 PER
1 ALCOHOL PREP PADS 10P
1 PVP IODINE WIPES 10 PER
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 FLEXICON 2"X 4.1 YD
1 FIRST AID GUIDE ASHI
1 ABD COMBINE PAD 5" X 9"
1 SCISSOR BDGE 4" RED PLS HDL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 LBL CONTS 6 3/4"X3 1/2" ID B
1 LABEL COVER, GRAINGER Z019819
1 KIT PP 24 UNIT FA
3 SAFETEC STING RELIEF WIPES BULK
1 FOILLE BURN/F A OINT 1/2 OZ
4 GAUZE PADS 2"X2" 12PLY
1 GAUZE PADS 4"X4" 12PLY
4 WOVEN FINGERTIP BANDAGE 2"
4 WOVEN KNUCKLE BANDAGE
4 HEAVY FLEX LARGE PATCH 2" X 3"
1 GAUZE PADS 3"X3" 4/BX
1 TRIANG 37X37X52 UNIT
8 AYPANAL EXTRA BULK 2/PK
-
4379
Z63158002 Kit Contents
1 TRIPLE ANTIBIOTIC 10 PER
1 INSTANT COLD PACK 4" X 6"
2 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 BURN JEL 1/8 OZ, 6 PER
1 ALCOHOL PREP PADS 10P
1 PVP IODINE WIPES 10 PER
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 FLEXICON 2"X 4.1 YD
1 FIRST AID GUIDE ASHI
1 ABD COMBINE PAD 5" X 9"
1 SCISSOR BDGE 4" RED PLS HDL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 LBL CONTS 6 3/4"X3 1/2" ID B
1 LABEL COVER, GRAINGER Z019819
1 KIT PP 24 UNIT FA
3 SAFETEC STING RELIEF WIPES BULK
1 FOILLE BURN/F A OINT 1/2 OZ
4 GAUZE PADS 2"X2" 12PLY
1 GAUZE PADS 4"X4" 12PLY
4 WOVEN FINGERTIP BANDAGE 2"
4 WOVEN KNUCKLE BANDAGE
4 HEAVY FLEX LARGE PATCH 2" X 3"
1 GAUZE PADS 3"X3" 4/BX
1 TRIANG 37X37X52 UNIT
8 AYPANAL EXTRA BULK 2/PK
- Triple Principal Display Panel
- Alcohol Principal Display Panel
- Foille Principal Display Panel
- Burn Jel Principal Display Panel
- Sting Relief Principal Display Panel
- PVP Principal Display Panel
- Aypanal EX Principal Display Panel
- BZK Principal Display Panel
- 4334 Kit Label Z019819
- 4373 Kit Label Z63158002
-
INGREDIENTS AND APPEARANCE
4379 FIRST AID KIT
4379 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4379 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4379-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 10 POUCH 4 mL Part 2 1 TUBE 14 g Part 3 6 PACKET 21 g Part 4 1 PACKET 1.4 mL Part 5 3 POUCH 1.2 mL Part 6 10 POUCH 3 mL Part 7 8 PACKET 16 Part 8 10 PACKET 9 g Part 1 of 8 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 2 of 8 BLISTEX FOILLE MEDICATED FIRST AID
benzocaine and chloroxylenol ointmentProduct Information Item Code (Source) NDC: 10157-9302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.1 g in 100 g BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM LAURYL SULFATE (UNII: 368GB5141J) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE CALCIUM DISODIUM ANHYDROUS (UNII: 8U5D034955) CERESIN (UNII: Q1LS2UJO3A) EUGENOL (UNII: 3T8H1794QW) MALEIC ANHYDRIDE (UNII: V5877ZJZ25) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) CORN OIL (UNII: 8470G57WFM) SODIUM BORATE (UNII: 91MBZ8H3QO) YELLOW WAX (UNII: 2ZA36H0S2V) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/05/2013 Part 3 of 8 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/19/2018 Part 4 of 8 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/21/2017 Part 5 of 8 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Part 6 of 8 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 7 of 8 AYPANAL EX
acetaminophen tabletProduct Information Item Code (Source) NDC: 0498-2110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code FR1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-2110-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 01/02/2017 Part 8 of 8 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC: 0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 4334 FIRST AID KIT
4334 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0498-4334 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-4334-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1.4 mL Part 2 3 POUCH 1.2 mL Part 3 10 POUCH 3 mL Part 4 8 PACKET 16 Part 5 10 PACKET 9 g Part 6 10 POUCH 4 mL Part 7 1 TUBE 14 g Part 8 6 PACKET 21 g Part 1 of 8 ANTISEPTIC TOWELETTE
benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 0498-0501 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0501-00 1.4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/21/2017 Part 2 of 8 STING RELIEF PAD
ethyl alcohol, lidocaine swabProduct Information Item Code (Source) NDC: 0498-0733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.5 mL in 1 mL Inactive Ingredients Ingredient Name Strength MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/23/2017 Part 3 of 8 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC: 0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 4 of 8 AYPANAL EX
acetaminophen tabletProduct Information Item Code (Source) NDC: 0498-2110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code FR1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-2110-01 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 01/02/2017 Part 5 of 8 TRIPLE ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate, neomycin sulfate ointmentProduct Information Item Code (Source) NDC: 0498-0750 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0750-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 09/19/2018 Part 6 of 8 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC: 0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 09/18/2018 Part 7 of 8 BLISTEX FOILLE MEDICATED FIRST AID
benzocaine and chloroxylenol ointmentProduct Information Item Code (Source) NDC: 10157-9302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.1 g in 100 g BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM LAURYL SULFATE (UNII: 368GB5141J) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE CALCIUM DISODIUM ANHYDROUS (UNII: 8U5D034955) CERESIN (UNII: Q1LS2UJO3A) EUGENOL (UNII: 3T8H1794QW) MALEIC ANHYDRIDE (UNII: V5877ZJZ25) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) CORN OIL (UNII: 8470G57WFM) SODIUM BORATE (UNII: 91MBZ8H3QO) YELLOW WAX (UNII: 2ZA36H0S2V) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/05/2013 Part 8 of 8 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC: 0498-0203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 g Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0498-0203-00 3.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (079287321) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 manufacture(10157-9302) Establishment Name Address ID/FEI Business Operations Honeywell Safety Products USA, INC 079287321 pack(0498-4334, 0498-4379) Establishment Name Address ID/FEI Business Operations Ultra Seal Corporation 085752004 manufacture(0498-2110) Establishment Name Address ID/FEI Business Operations Water-Jel Technologies 155522589 manufacture(0498-0750, 0498-0203) Establishment Name Address ID/FEI Business Operations Changzhou Maokang Medical 421317073 manufacture(0498-0143, 0498-0501) Establishment Name Address ID/FEI Business Operations Sion Medical Biotext 532775194 manufacture(0498-0121) Establishment Name Address ID/FEI Business Operations Safetec of America Inc 874965262 manufacture(0498-0733)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.