DIBUCAINE by E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. DIBUCAINE ointment
DIBUCAINE by
Drug Labeling and Warnings
DIBUCAINE by is a Otc medication manufactured, distributed, or labeled by E. Fougera & Co. a division of Fougera Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient:
- Purpose:
- INDICATIONS & USAGE
- WARNINGS
- Keep out of reach of children.
- DOSAGE & ADMINISTRATION
- SPL UNCLASSIFIED SECTION
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
-
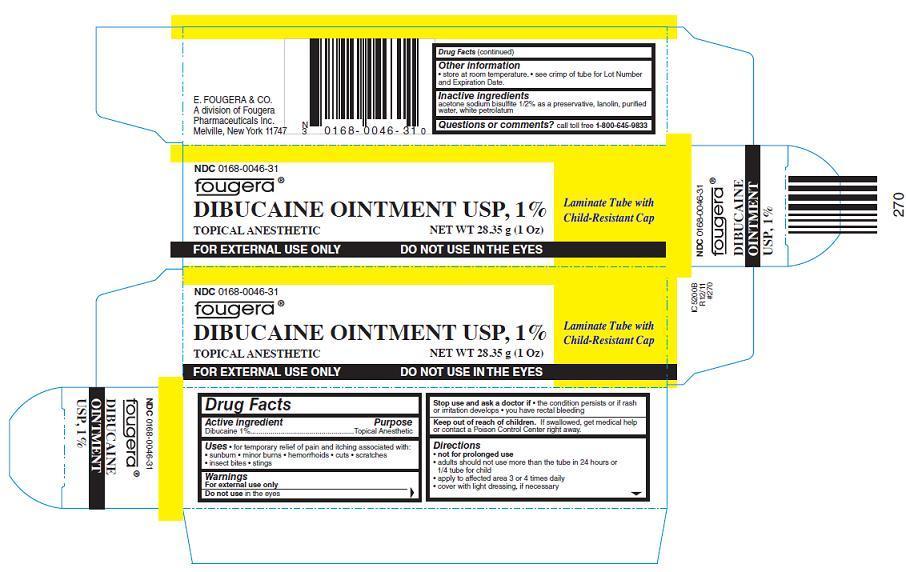
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – CONTAINER
Fougera®
NDC: 0168-0046-31
CHILD-RESISTANT PACKAGE
DIBUCAINE OINTMENT USP, 1%
TOPICAL ANESTHETIC
NET WT 28.35g (1 Oz)
FOR EXTERNAL USE ONLY
DO NOT USE IN THE EYES
-
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – CARTON
NDC: 0168-0046-31
Fougera®
DIBUCAINE OINTMENT USP, 1%
TOPICAL ANESTHETIC
NET WT 28.35g (1 Oz)
Laminate Tube with Child-Resistant Cap
FOR EXTERNAL USE ONLY
DO NOT USE IN THE EYES
-
INGREDIENTS AND APPEARANCE
DIBUCAINE
dibucaine ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0168-0046 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIBUCAINE (UNII: L6JW2TJG99) (DIBUCAINE - UNII:L6JW2TJG99) DIBUCAINE 1 g in 100 g Inactive Ingredients Ingredient Name Strength ACETONE SODIUM BISULFITE (UNII: 47VY054OXY) LANOLIN (UNII: 7EV65EAW6H) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0168-0046-31 1 in 1 CARTON 1 28 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 01/01/1968 Labeler - E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. (043838424)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.