PHOSPHOLINE IODIDE- echothiophate iodide for ophthalmic solution kit
Phospholine Iodide by
Drug Labeling and Warnings
Phospholine Iodide by is a Prescription medication manufactured, distributed, or labeled by Fera Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
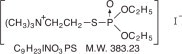
Chemical name: (2-mercaptoethyl) trimethylammonium iodide O,O-diethyl phosphorothioate
Structural formula
Echothiophate iodide for ophthalmic solution occurs as a white, crystalline, water-soluble, hygroscopic solid having a slight mercaptan-like odor. When freeze-dried in the presence of potassium acetate, the mixture appears as a white amorphous deposit on the walls of the bottle.
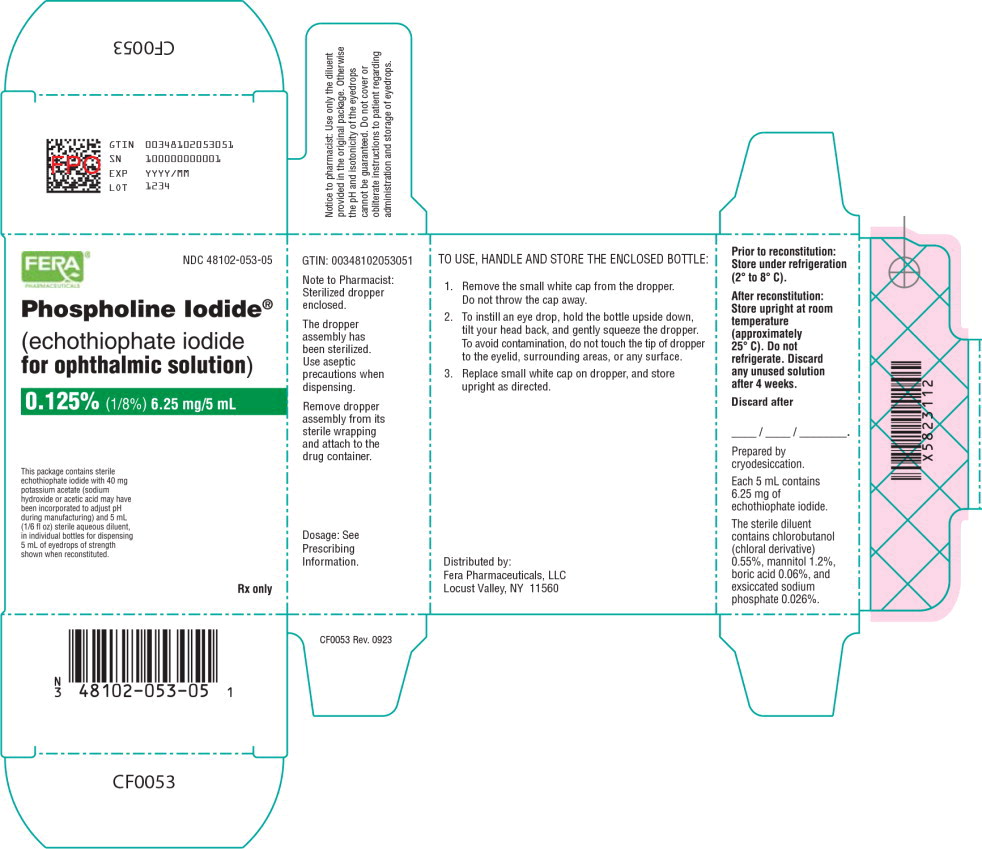
Each package contains materials for dispensing 5 mL of eyedrops: (1) bottle containing sterile echothiophate iodide for ophthalmic solution 6.25 mg (0.125%) with 40 mg potassium acetate. Sodium hydroxide or acetic acid may have been incorporated to adjust pH during manufacturing; (2) a 5 mL bottle of sterile diluent containing chlorobutanol (chloral derivative), 0.55%; mannitol, 1.2%; boric acid, 0.06%; and sodium phosphate, 0.026%; (3) sterilized dropper.
-
CLINICAL PHARMACOLOGY
Echothiophate iodide for ophthalmic solution is a long-acting cholinesterase inhibitor for topical use which enhances the effect of endogenously liberated acetylcholine in iris, ciliary muscle, and other parasympathetically innervated structures of the eye. It thereby causes miosis, increase in facility of outflow of aqueous humor, fall in intraocular pressure (IOP), and potentiation of accommodation.
Echothiophate iodide for ophthalmic solution will depress both plasma and erythrocyte cholinesterase levels in most patients after a few weeks of eyedrop therapy.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
- Succinylcholine should be administered only with great caution, if at all, prior to or during general anesthesia to patients receiving anticholinesterase medication because of possible respiratory or cardiovascular collapse.
- Caution should be observed in treating elevated IOP with echothiophate iodide for ophthalmic solution in patients who are at the same time undergoing treatment with systemic anticholinesterase medications, because of possible adverse additive effects.
-
PRECAUTIONS
General
- Digital compression of the nasolacrimal ducts for a minute or two following instillation to minimize drainage into the nasal chamber is recommended. To prevent possible skin absorption, hands should be washed following instillation.
- Discontinue use of the medication if cardiac irregularities occur.
- Anticholinesterase drugs should be used with caution, if at all, in patients with marked vagotonia, bronchial asthma, spastic gastrointestinal disturbances, peptic ulcer, pronounced bradycardia and hypotension, recent myocardial infarction, epilepsy, parkinsonism, and other disorders that may respond adversely to vagotonic effects.
- Echothiophate iodide for ophthalmic solution should be used with caution, where there is a prior history of retinal detachment.
- Temporary discontinuance of medication is necessary if salivation, urinary incontinence, diarrhea, profuse sweating, muscle weakness, or respiratory difficulties occur.
- Patients receiving echothiophate iodide for ophthalmic solution who are exposed to carbamate- or organophosphate-type insecticides and pesticides should be warned of the additive systemic effects possible from absorption of the pesticide through the respiratory tract or skin. During periods of exposure to such pesticides, the wearing of respiratory masks, and frequent washing and clothing changes may be advisable.
Drug Interactions
Echothiophate iodide for ophthalmic solution potentiates other cholinesterase inhibitors such as succinylcholine or organophosphate and carbamate insecticides. Patients undergoing systemic anticholinesterase treatment should be warned of the possible additive effects of echothiophate iodide for ophthalmic solution.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No data is available regarding carcinogenesis, mutagenesis, and impairment of fertility.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with echothiophate iodide for ophthalmic solution. It is also not known whether echothiophate iodide for ophthalmic solution can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Echothiophate iodide for ophthalmic solution should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
- Although the relationship, if any, of retinal detachment to the administration of echothiophate iodide for ophthalmic solution has not been established, retinal detachment has been reported in a few cases during the use of echothiophate iodide for ophthalmic solution in adult patients without a previous history of this disorder.
- Stinging, burning, lacrimation, lid muscle twitching, conjunctival and ciliary redness, browache, induced myopia with visual blurring may occur.
- Activation of latent iritis or uveitis may occur.
- Iris cysts may form, and if treatment is continued, may enlarge and obscure vision. This occurrence is more frequent in children. The cysts usually shrink upon discontinuance of the medication or by reducing the frequency of instillation. Rarely, they may rupture or break free into the aqueous. Regular examinations are advisable when the drug is being prescribed for the treatment of accommodative esotropia.
- Prolonged use may cause conjunctival thickening, obstruction of nasolacrimal canals.
- Lens opacities have been reported with echothiophate iodide.
- Paradoxical increase in IOP may follow anticholinesterase instillation. This may be alleviated by prescribing a sympathomimetic mydriatic such as phenylephrine.
- Cardiac irregularities.
- DOSAGE AND ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
Directions for Preparing Eyedrops
- Use aseptic technique.
- Tear off aluminum seals and remove and discard rubber plugs from both drug and diluent containers.
- Pour diluent into drug container.
- Remove dropper assembly from its sterile wrapping and attach to the drug container.
- Shake for several seconds to ensure mixing.
- Do not cover nor obliterate instructions to patient regarding administration and storage of eyedrops.
Elevated IOP
Echothiophate iodide for ophthalmic solution one drop instilled twice a day, just before retiring and in the morning. A change in therapy is indicated if, at any time, the tension fails to remain at an acceptable level on this regimen.
Two doses a day are preferred to one in order to maintain as smooth a diurnal tension curve as possible, although a single dose per day or every other day has been used with satisfactory results. The daily dose or one of the two daily doses should always be instilled just before retiring to avoid inconvenience due to the miosis.
Technique – Technique in the administration of echothiophate iodide for ophthalmic solution may include finger pressure at the inner canthus exerted for a minute or two following instillation of the eyedrops, to potentially minimize drainage into the nose and throat. Excess solution around the eye should be removed with tissue and any medication on the hands should be rinsed off.
Accommodative Esotropia (Pediatric Use)
In Diagnosis – One drop may be instilled once a day in both eyes on retiring, for a period of two or three weeks. If the esotropia is accommodative, a favorable response will usually be noted which may begin within a few hours.
In Treatment – Echothiophate iodide for ophthalmic solution is prescribed at the lowest frequency which gives satisfactory results. After the initial period of treatment for diagnostic purposes, the schedule may be reduced to one drop every other day. The maximum usually recommended dosage is one drop once a day, although more intensive therapy has been used for short periods.
Technique – (See “DOSAGE AND ADMINISTRATION”)
Duration of Treatment — In therapy, there is no definite limit so long as the drug is well tolerated. However, if the eyedrops, with or without eyeglasses, are gradually withdrawn after about a year or two and deviation recurs, surgery should be considered. As with other miotics, tolerance may occasionally develop after prolonged use. In such cases, a rest period may restore the original activity of the drug.
-
HOW SUPPLIED
Each package contains sterile echothiophate iodide for ophthalmic solution, sterile diluent, and dropper for dispensing 5 mL eyedrops.
NDC: 48102-053-05 ................... 6.25 mg package for 0.125%
White amorphous deposit on bottle walls. Aluminum crimp seal is green.
HANDLING AND STORAGE:
Prior to reconstitution: Store under refrigeration (2°C to 8°C).
After reconstitution: Store upright at room temperature (approximately 25°C)
(68°F to 77° F).
Do not refrigerate. Discard any unused solution after 4 weeks.
FERA®
PHARMACEUTICALS
Distributed by:
Fera Pharmaceuticals, LLC
Locust Valley, NY 11560
PF053Rev. 0923
-
Principal Display Panel - 6.25 mg/5 mL Carton Label
FERA®
PHARMACEUTICALSNDC: 48102-053-05
Phospholine Iodide®
(echothiophate iodide
for ophthalmic solution)0.125% (1/8%) 6.25 mg/5 mL
This package contains sterile
echothiophate iodide with 40 mg
potassium acetate (sodium
hydroxide or acetic acid may have
been incorporated to adjust pH
during manufacturing) and 5 mL
(1/6 fl oz) sterile aqueous diluent,
in individual bottles for dispensing
5 mL of eyedrops of strength
shown when reconstituted.Rx only
-
Principal Display Panel - 6.25 mg/5 mL Bottle Label
NDC: 48102-054-05
Rx only
Phospholine Iodide®
(echothiophate iodide
for ophthalmic solution)0.125% (1/8%) 6.25 mg/5 mL
Each 5 mL contains 6.25 mg of echothiophate iodide
With 40 mg Potassium Acetate for preparing 5 mL eyedrops.See Prescribing Information.
-
Principal Display Panel - 5 mL Bottle Label
NDC: 48102-055-05
STERILE DILUENT
FOR PREPARING
Phospholine Iodide®(echothiophate iodide
for ophthalmic solution)
5 mL (1/6 fl oz)
EYEDROPS
-
INGREDIENTS AND APPEARANCE
PHOSPHOLINE IODIDE
echothiophate iodide for ophthalmic solution kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 48102-053 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48102-053-05 1 in 1 CARTON; Type 0: Not a Combination Product 06/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 6.25 mL Part 2 1 BOTTLE 5 mL Part 1 of 2 PHOSPHOLINE IODIDE
echothiophate iodide powder, for solutionProduct Information Item Code (Source) NDC: 48102-054 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength echothiophate iodide (UNII: BA9QH3P00T) (echothiophate - UNII:0F350BVT6S) echothiophate iodide 6.25 mg in 5 mL Inactive Ingredients Ingredient Name Strength potassium acetate (UNII: M911911U02) 40 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48102-054-05 6.25 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA011963 06/01/2022 Part 2 of 2 STERILE DILUENT FOR PREPARING PHOSPHOLINE IODIDE
diluent solution solutionProduct Information Item Code (Source) NDC: 48102-055 Route of Administration OPHTHALMIC Inactive Ingredients Ingredient Name Strength chlorobutanol (UNII: HM4YQM8WRC) 27.5 mg in 5 mL mannitol (UNII: 3OWL53L36A) 60 mg in 5 mL boric acid (UNII: R57ZHV85D4) 3 mg in 5 mL sodium phosphate (UNII: SE337SVY37) 1.3 mg in 5 mL water (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48102-055-05 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA011963 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA011963 06/01/2022 Labeler - Fera Pharmaceuticals, LLC (831023713)
Trademark Results [Phospholine Iodide]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PHOSPHOLINE IODIDE 72087782 0704866 Live/Registered |
CAMPBELL PHARMAMEUTICALS, INC. 1959-12-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.