MUCINEX- guaifenesin tablet, extended release
Mucinex by
Drug Labeling and Warnings
Mucinex by is a Otc medication manufactured, distributed, or labeled by Cardinal Health 107, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each extended-release bi-layer tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
This Unit Dose package is not child resistant and is Intended for Institutional Use Only. KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDREN.
If dispensed for outpatient use, a child-resistant container should be utilized.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
SPL UNCLASSIFIED SECTION
* The name MUCINEX® and associated trademarked items are used with permission by Reckitt Benckiser
The drug product contained in this package is from NDC # 63824-008 Reckitt Benckiser.
Packaged and Distributed by American Health Packaging, 2550 John Glenn Avenue, Suite A, Columbus, OH 43217
Americanhealthpackaging.com
An AmerisourceBergen Company
Distributed by:
Cardinal Health
Dublin, OH 43017
057201
5500408/0915L5315882-10318
L5315882-20318
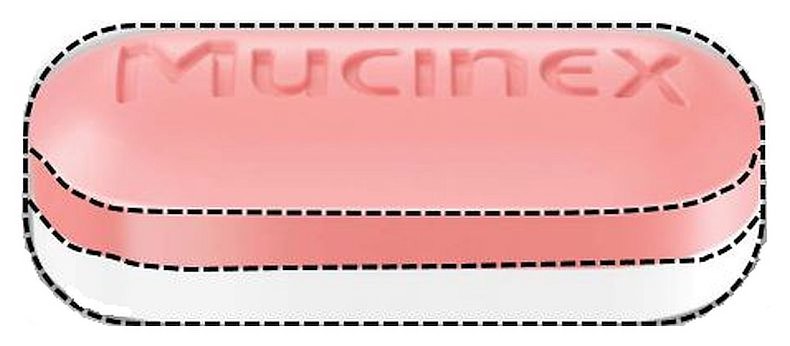
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MUCINEX
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55154-4680(NDC:68084-572) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white (blue and white) Score no score Shape OVAL Size 16mm Flavor Imprint Code Mucinex;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55154-4680-0 10 in 1 CARTON 01/01/2016 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021282 01/01/2016 Labeler - Cardinal Health (603638201)
Trademark Results [Mucinex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCINEX 98499792 not registered Live/Pending |
RB Health (US) LLC 2024-04-15 |
 MUCINEX 90229382 not registered Live/Pending |
RB Health (US) LLC 2020-10-01 |
 MUCINEX 88040437 not registered Live/Pending |
Reckitt Benckiser LLC 2018-07-17 |
 MUCINEX 86191608 4613098 Live/Registered |
RB HEALTH (US) LLC 2014-02-12 |
 MUCINEX 85496330 4168070 Live/Registered |
RB HEALTH (US) LLC 2011-12-15 |
 MUCINEX 85489681 4168052 Live/Registered |
RB HEALTH (US) LLC 2011-12-07 |
 MUCINEX 77770135 3722363 Live/Registered |
Reckitt Benckiser Inc. 2009-06-29 |
 MUCINEX 76297961 2670161 Live/Registered |
RB HEALTH (US) LLC 2001-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
