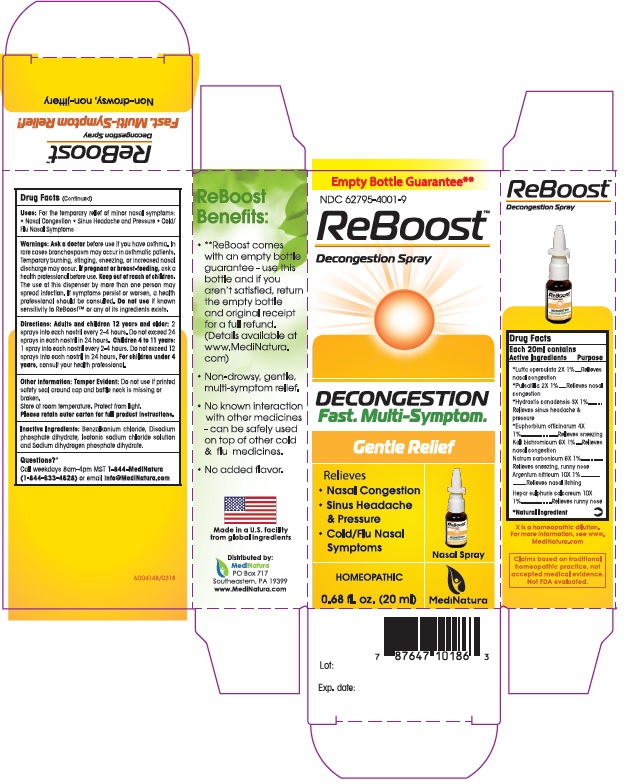
ReBoost by MediNatura ReBoost Nasal Spray
ReBoost by
Drug Labeling and Warnings
ReBoost by is a Homeopathic medication manufactured, distributed, or labeled by MediNatura. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REBOOST- silver nitrate,euphorbia resinifera resin, calcium sulfide, goldenseal, potassium dichromate,luffa operculata fruit, sodium carbonate, and pulsatilla vulgaris spray
MediNatura
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ReBoost Nasal Spray
ACTIVE INGREDIENTS
Each 20ml contains
Active Ingredients Purpose
Argentum nitricum10X................Relieves nasal itching
*Euphorbium officinarum 4X........Relieves sneezing
Hepar sulphuris calcareum 10X....Relieves runny nose
*Hydrastis canadensis 3X............Relieves sinus headache & pressure
Kali bichromicum 6X....................Relieves nasal congestion
*Luffa operculata 2X...................Relieves nasal congestion
Natrum carbonicum 6X...............Relieves sneezing and runny nose
*Pulsatilla 2X..............................Relieves nasal congestion
*Natural Ingredients
Uses
For the temporary relief of minor nasal symptoms:Nasal Congestion Sinus Headache and Pressure Cold/Flu Nasal Symptoms
WARNINGS
Warnings: Ask a doctor before use if you have asthma. In rare cases bronchospasm may occur in asthmatic patients. Temporary burning, stinging, sneezing, or increased nasal discharge may occur. If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. The use of this dispenser by more than one person may spread infection. If symptoms persist or worsen, a health professional should be consulted. Do not use if known sensitivity to ReBoost™ or any of its ingredients exists.
DIRECTIONS
At first sign of symptoms: Adults and children12 years and older: 2 sprays into each nostril every ½ to 1 hour, until symptoms lessen, then continue with standard dosage. Do not exceed 48 sprays in 24 hours.
Children 4 to 11 years: 1 spray into each nostril every ½ to 1 hour until symptoms lessen, then continue with standard dosage.
Do not exceed 24 sprays in 24 hours.
For children under 4 years, consult your health professional.
Standard Dosage:Adults and children 12 years and older: 2 sprays into each nostril every 4 to 6 hours. Children 4 to 11 years: 1 spray into each nostril every 4 to 6 hours. For children under 4 years, consult your health professional.
| REBOOST
silver nitrate,euphorbia resinifera resin, calcium sulfide, goldenseal, potassium dichromate,luffa operculata fruit, sodium carbonate, and pulsatilla vulgaris spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - MediNatura (079324099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MediNatura | 102783016 | manufacture(62795-4001) | |
Trademark Results [ReBoost]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REBOOST 86301671 4805108 Live/Registered |
MediNatura Inc. 2014-06-05 |
 REBOOST 86274593 not registered Dead/Abandoned |
Select Homes, LLC 2014-05-07 |
 REBOOST 85974408 not registered Dead/Abandoned |
Biologische Heilmittel Heel GmbH 2013-07-01 |
 REBOOST 85648498 not registered Dead/Abandoned |
Heel Inc. 2012-06-11 |
 REBOOST 77475902 3655677 Live/Registered |
SEELECT, INC. 2008-05-15 |
 REBOOST 75907343 2433396 Dead/Cancelled |
SmithKline Beecham Biologicals, S.A. 2000-01-19 |
 REBOOST 75308717 not registered Dead/Abandoned |
SmithKline Beecham Biologicals S.A. 1997-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.