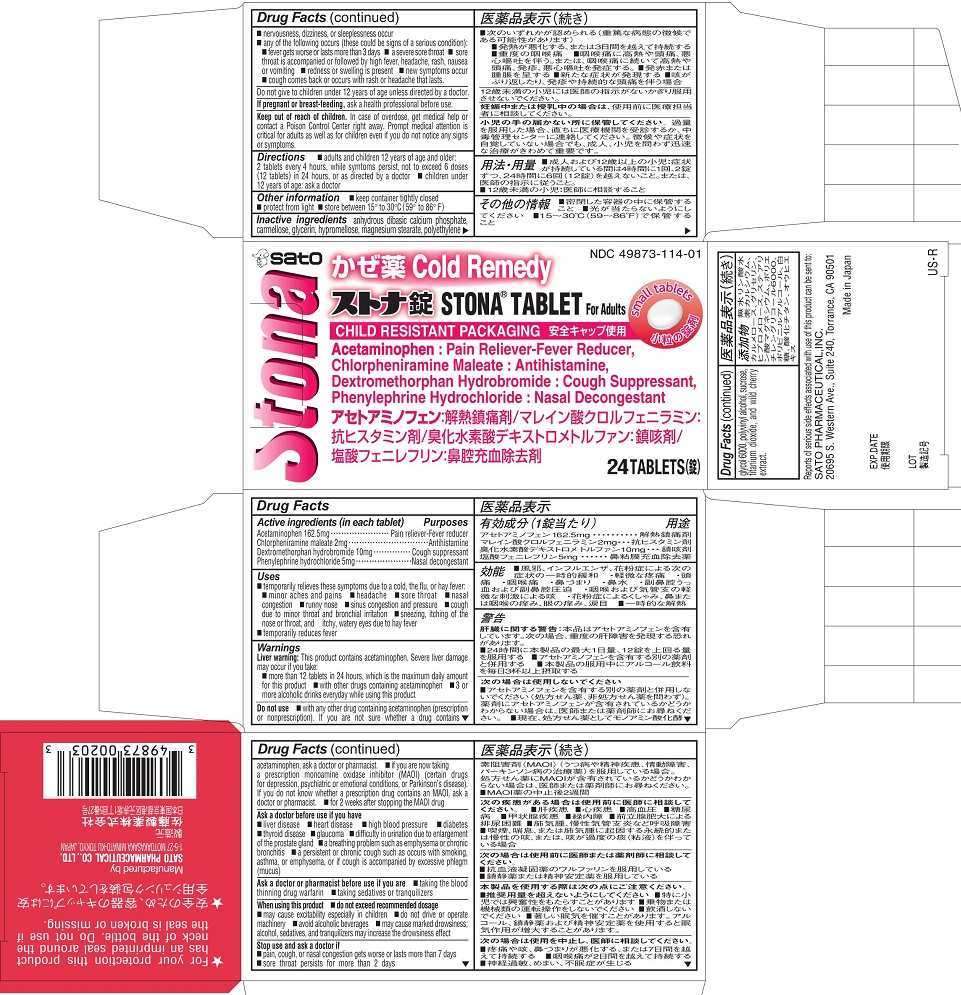
STONA- acetaminophen, chlorpheniramine maleate, dextromethorphan hydrobromide, phenylephrine hydrochloride tablet
Stona by
Drug Labeling and Warnings
Stona by is a Otc medication manufactured, distributed, or labeled by Sato Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
■ temporarily relieves these symptoms due to a cold, the flu, or hay fever:
■ minor aches and pains ■ headache ■ sore throat ■ nasal congestion
■ runny nose ■ sinus congestion and pressure
■ cough due to minor throat and bronchial irritation
■ sneezing, itching of the nose or throat, and itchy, watery eyes due to hay fever
■ temporarily reduces fever
-
WARNINGS
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
■ more than 12 tablets in 24 hours, which is the maximum daily amount for this product
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks everyday while using this productDo not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease). If you do not know whether a prescription drug contains an MAOI, ask a doctor or pharmacist.
■ for 2 weeks after stopping the MAOI drugAsk a doctor before use if you have
■ liver disease ■ heart disease ■ high blood pressure ■ diabetes
■ thyroid disease ■ glaucoma ■ difficulty in urination due to enlargement of the prostate gland
■ a breathing problem such as emphysema or chronic bronchitis
■ a persistent or chronic cough such as occurs with smoking, asthma, or emphysema, or if cough is accompanied by excessive phlegm (mucus)
Ask a doctor or pharmacist before use if you are
■ taking the blood thinning drug warfarin ■ taking sedatives or tranquilizersWhen using this product
■ do not exceed recommended dosage
■ may cause excitability especially in children■ do not drive or operate machinery
■ avoid alcoholic beverages
■ may cause marked drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effectStop use and ask a doctor if
■ pain, cough, or nasal congestion gets worse or lasts more than 7 days
■ sore throat persists for more than 2 days
■ nervousness, dizziness, or sleeplessness occur
■ any of the following occurs (these could be signs of a serious condition):■ fever gets worse or or lasts more than 3 days
■ a severe sore throat
■ sore throat is accompanied or followed by high fever, headache, rash, nausea or vomiting
■ redness or swelling is present■ new symptoms occur
■ cough comes back or occurs with rash or headache that lasts - DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STONA
acetaminophen, chlorpheniramine maleate, dextromethorphan hydrobromide, phenylephrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49873-114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 162.5 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) POLYVINYL ALCOHOL (UNII: 532B59J990) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 10mm Flavor CHERRY (WILD CHERRY EXTRACT) Imprint Code SATO;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49873-114-01 1 in 1 CARTON 09/29/2004 1 24 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/29/2004 Labeler - Sato Pharmaceutical Co., Ltd. (690575642) Establishment Name Address ID/FEI Business Operations MeriCal, Inc. 029644978 pack(49873-114) , label(49873-114) Establishment Name Address ID/FEI Business Operations Sato Pharmaceutical Co., Ltd. 715699133 manufacture(49873-114)
Trademark Results [Stona]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STONA 90215483 not registered Live/Pending |
Shen,Yahui 2020-09-27 |
 STONA 90102550 not registered Live/Pending |
Shen,Yahui 2020-08-10 |
 STONA 90059262 not registered Live/Pending |
Stona GmbH 2020-07-17 |
 STONA 86171506 5193661 Live/Registered |
PERDURA STONE, S.A. de C.V. 2014-01-21 |
 STONA 76354210 2772365 Live/Registered |
Sato Pharmaceutical Co., Ltd. 2001-12-31 |
 STONA 73380117 1289675 Live/Registered |
Sato Pharmaceutical Co., Ltd. 1982-08-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.