Bali Body Peach Tanning Oil SPF 6
Bali Body SPF 15 Cacao Tanning Oil by
Drug Labeling and Warnings
Bali Body SPF 15 Cacao Tanning Oil by is a Otc medication manufactured, distributed, or labeled by Bali Body Pty Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BALI BODY SPF 15 CACAO TANNING OIL- octinoxate, octocrylene cream oil
Bali Body Pty Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bali Body Peach Tanning Oil SPF 6
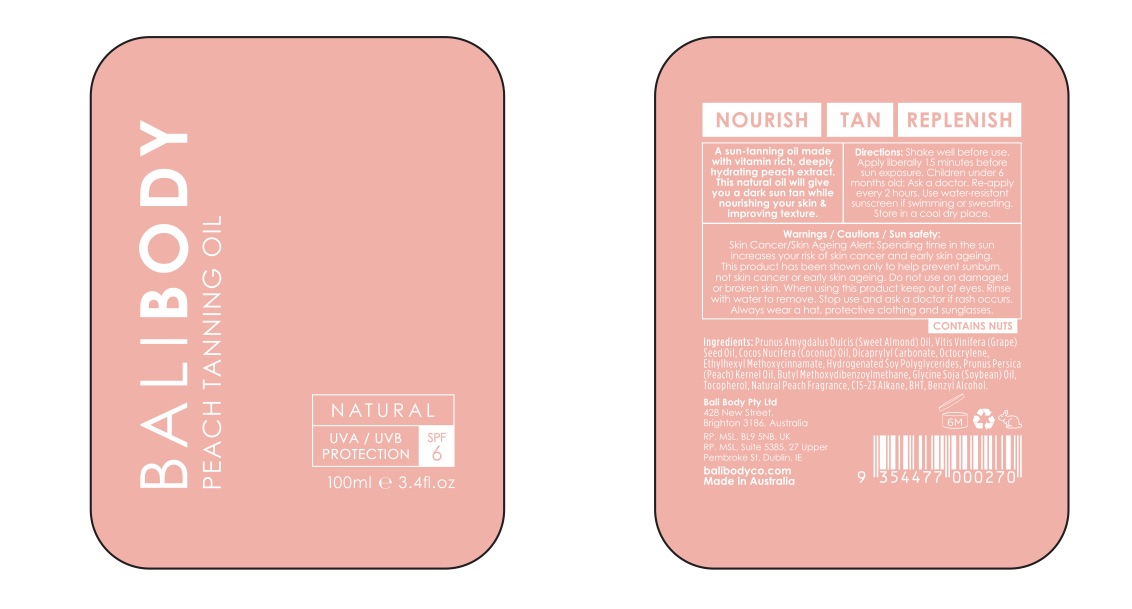
Shake well before use. Apply liberaly 15 minutes before sun exposure.
Children under 6 months old: Ask a doctor.
Re-apply every 2 hours.
Use water-resistant sunscreen if swimming or sweating.
Store in cool dry place.
Vitis Vinifera (Grape) seed oil
Cocos Nucifera Oil (Coconut)
Sweet Almond Oil
Vitamin E
Dicaprylyl Carbonate
Hydrogenated Soy Polyglycerides
Soybean Oil
C15-23 Alkane
BHT
Benzyl Alcohol
Skin Cancer/Skin Ageing Alert: Spending time in the sun increases your risk of skin cancer and early skin aging.
This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Always wear a hat, protective clothing and sunglasses.
| BALI BODY SPF 15 CACAO TANNING OIL
octinoxate, octocrylene cream oil |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Bali Body Pty Ltd (757840223) |
| Registrant - Bali Body Pty Ltd (757840223) |